Egypt Liquid Biopsy Market Analysis
Egypt Liquid Biopsy Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Liquid Biopsy is expanding as a result of rising healthcare spending in both developed and developing nations. This demand is fueling the development of better medical instruments and methods for the accurate diagnosis and treatment of various medical conditions, including cancer. Some of the key players in the global Liquid Biopsy Market include Hoffmann-La Roche Ltd, Guardant Health, Thermo Fisher Scientific Inc., Exact Sciences Corporation (Genomic Health), QIAGEN, Labcorp, Johnson & Johnson Services, Inc., Illumina Inc, Mdxhealth, NeoGenomics Laboratories, Bio-Rad Laboratories, Inc., Natera, Inc., Cardiff Oncology, INC., PathAI, Streck, Menarini Silicon Biosystems, and Biocept, Inc.
Buy Now

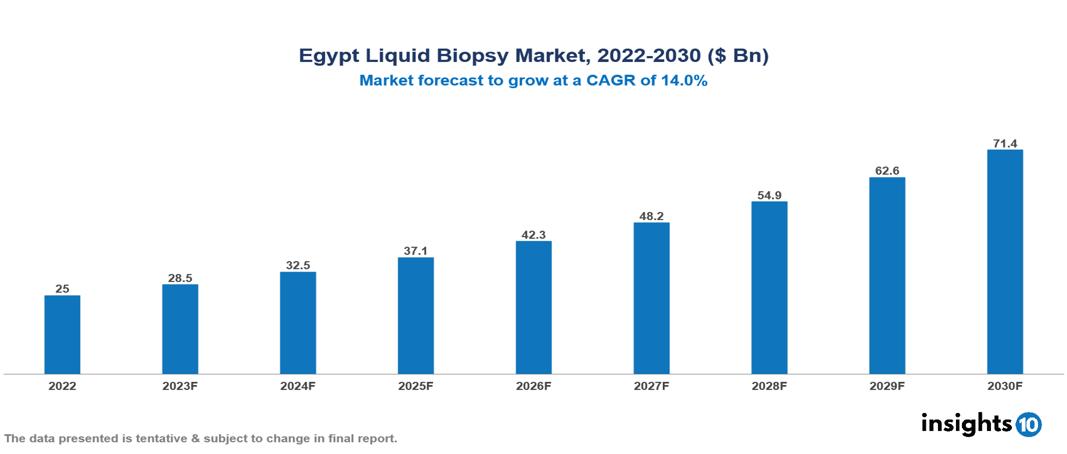
Egypt Liquid Biopsy Market Executive Summary
Egypt Liquid Biopsy Market is valued at around $25 Bn in 2022 and is projected to reach $71.2 Bn by 2030, exhibiting a CAGR of 14% during the forecast period 2023-2030.
The Liquid Biopsy Market refers to the branch of diagnostic testing which examines and analyzes biomarkers in different body fluids like blood, urine, and cerebrospinal fluid to diagnose various medical conditions including cancer. It has ability to detect genetic mutations and other molecular changes. It is a minimally invasive procedure and provides real-time monitoring.
The increased prevalence of cancer has contributed to the growth of the global market for liquid biopsy in recent years. According to WHO around 19.2 Mn people worldwide were suffering from various types of cancer in 2022. Prevalence is increasing with every passing year and the most common type of cancers include breast cancer, lung cancer, colorectal cancer, prostate cancer, and skin cancer. It is increasing demand for advanced and improved technology and methods for cancer diagnosis and treatment, driving the market for liquid biopsy.
There are many global players in the liquid biopsy market but Hoffmann-La Roche Ltd, Guardant Health, Thermo Fisher Scientific Inc., Exact Sciences Corporation (Genomic Health), QIAGEN, Labcorp, Johnson & Johnson Services, Inc., Illumina Inc, mdxhealth, NeoGenomics Laboratories, Bio-Rad Laboratories, Inc., Natera, Inc., Cardiff Oncology, INC., PathAI, Streck, Menarini Silicon Biosystems, and Biocept, Inc. are some of the major players in market.
Liquid biopsy has the potential to revolutionize the way diagnosis and treatment of cancer have been done till now. It is more accurate than any other diagnostic method including imaging tests. Liquid biopsy can be used to monitor the progress of treatment and the prognosis of treatment. CTCs and cfDNA are the most commonly used and major liquid biopsy techniques. The field of liquid biopsy is evolving and being improved with new advancements.
The increasing prevalence of cancer and other medical conditions, demand for non-invasive procedures, and increasing healthcare expenditure are projected to fuel the market for liquid biopsy in the coming years. The market still has many challenges like complex regulatory procedures, high cost of equipment and setup, and lack of skilled professionals.
Market Dynamics
Drivers of Egypt Liquid Biopsy Market:
Growing Incidence and Prevalence of Cancer: The prevalence of cancer is increasing with time due to factors like changes in lifestyle, environmental factors like pollution and radiation, and the aging population. This is raising the demand for accurate and effective methods and devices for diagnosis and treatment of various types of cancer. It is driving the market for liquid biopsy.
Non-Invasive Procedures: Demand for non-invasive procedures is increasing with time. Liquid biopsies taken from cancer patients have made it possible to identify new biomarkers for drug resistance. It performs continuous monitoring and treatment based on precision medicine. There is an increasing need for non-invasive cancer diagnostics since they provide a safer and more patient-friendly approach.
Increasing Healthcare Expenditure: The market for Liquid Biopsy is expanding as a result of rising healthcare spending in both developed and developing nations. This demand is fueling the development of better diagnostic tools and methods for the accurate diagnosis and treatment of cancer.
Advancement in Precision Medicine: Liquid biopsy has made it possible to make advancements in precision medicine. In order to enable precision medicine methods in cancer treatment, liquid biopsy techniques, such as the examination of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), or exosomes in blood or other bodily fluids, provide important knowledge on the genetic makeup of tumors.
Restraints of Egypt Liquid Biopsy Market:
High Cost: The cost of liquid biopsy is much higher than conventional biopsy and it can affect the affordability of tests in people with lower disposable income. It has limited the accessibility and affordability of the patients as well as small healthcare setups because of the higher cost of equipment and reagents. It can poise the growth of the market for liquid biopsy.
Limited Biomarker Coverage: Primary focus of liquid biopsy is to detect genetic alterations which can be a limitation in coverage of biomarkers. Liquid biopsy can not capture all the heterogenicity present in the cancer and all biomarkers may not be present in body fluids. It can limit the growth of the market for liquid biopsy.
Notable Deals in Liquid Biopsy Market
In January 2022, Guardant Health declared that the FDA had granted Breakthrough Device Designation for its Guardant360 CDx test for patients with advanced solid tumors. The FDA Breakthrough Device Designation program aims to hasten the creation and evaluation of medical devices that have the potential to significantly advance the diagnosis or treatment of fatal or crippling diseases.
In March 2022, The FDA authorized the DetermaVu Prostate Test. The DetermaVu Prostate Test is a blood test that scans the blood for DNA snippets from prostate cancer cells. When a man's prostate-specific antigen (PSA) level is raised, the test is meant to be a diagnostic aid for prostate cancer.
In April 2022, a study released an issue of the journal Nature Medicine discovered that a liquid biopsy test known as the CancerSEEK test has a high degree of accuracy in identifying early-stage lung cancer. In a blood test called CancerSEEK, eight distinct cancer-related indicators are sought after. The study discovered that the test has a 90% accuracy rate in identifying early-stage lung cancer.
Key players
Cairo Scan Radiology Centers Al Borg Laboratories Dar Al Fouad Hospital Al-Mokhtabar Medical BioLab NODCAR Prime Speed Medical Laboratory BioMerieux Egypt CEDARS Abu Dhabi Medical Industries (AMI)1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Egypt Liquid Biopsy Market
By Clinical Application:
- Routine Screening
- Therapy Selection
- Treatment Monitoring
- Recurrence Monitoring
- Patient Work-Up
- Others
By Technology:
- Multi-Gene-Parallel Analysis
- Single Gene Analysis
By End-User:
- Hospitals
- Reference Laboratories
- Diagnostics Centers
- Research Centers and Academic Institutes
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































