Egypt Leukocyte Adhesion Deficiency (LAD-I) Market Analysis
Egypt Leukocyte Adhesion Deficiency (LAD-I) therapeutics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. An uncommon genetic condition called leukocyte adhesion deficiency (LAD-I) impairs the immune system's capacity to combat infections. There is currently no cure for LAD-I, and there are few effective treatments available. To fill this unmet medical need, new medicines are becoming more and more popular. Vertex Pharmaceuticals, Orpha Labs, Avalo Therapeutics, Inc., Rocket Pharmaceuticals, Inc., Aspen Neuroscience, Magenta Therapeutics, Rubius Therapeutics, Enochian Biosciences, Sana Biotechnology, and Sigma-Aldrich are major global players.
Buy Now

Egypt Leukocyte Adhesion Deficiency (LAD-I) Market Analysis Summary
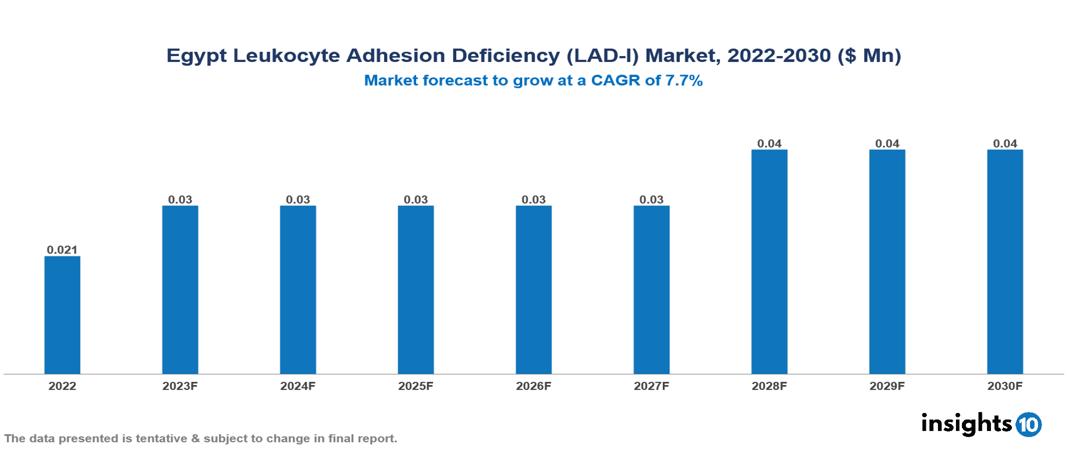
Egypt Leukocyte Adhesion Deficiency (LAD-I) Market is valued at around $0.021 Mn in 2022 and is projected to reach $0.04 Mn by 2030, exhibiting a CAGR of 7.7% during the forecast period 2023-2030.
An uncommon autosomal recessive paediatric condition called Leukocyte Adhesion Deficiency-I (LAD-I) is brought on by mutations in the ITGB2 gene, which codes for the protein CD18, a component of beta-2 integrins. Leukocyte adhesion and extravasation from blood arteries to fight infections are made possible by the crucial protein CD18. Children with severe LAD-I therefore frequently have symptoms soon after birth. They experience repeated, sometimes fatal bacterial and fungal infections during infancy, which necessitate many hospitalizations and poor treatment response. Recurrent severe infections in infants who survive infancy include pneumonia, gingival ulcers, necrotic skin ulcers, and septicemia. Patients with severe LAD-I have a death rate of 60–75 per cent before the age of 2, and survival past the age of five is unusual without a successful bone marrow transplant. Patients with severe LAD-I have a large unmet medical need.
There is currently no cure for LAD-I, and there are few effective treatments available. To fill this unmet medical need, new medicines are becoming more and more popular. There is optimism that novel medicines for LAD-I will be licensed soon given the rising interest in rare diseases and the expanding number of incentives provided by regulatory agencies.
Vertex Pharmaceuticals, Orpha Labs, Avalo Therapeutics, Inc., Rocket Pharmaceuticals, Inc., Aspen Neuroscience, Magenta Therapeutics, Rubius Therapeutics, Enochian Biosciences, Sana Biotechnology, and Sigma-Aldrich are major global players.
Market Dynamics
Market Drivers
There is currently no cure for LAD-I, and there are few effective treatments available. To fill this unmet medical need, new medicines are becoming more and more popular. Currently, a number of businesses are working to develop gene therapies and biologics as LAD-I treatments. However, there is hope that new treatments for LAD-I will be approved soon due to the rising interest in rare diseases and the growing number of incentives provided by regulatory agencies. Therefore, the LAD-I market offers a compelling prospect for businesses willing to make an investment in this field.
Market Development
- For severe Leukocyte Adhesion Deficiency-I, Rocket's ex vivo lentiviral gene therapy candidate is called RP-L201 (LAD-I).
- All nine LAD-I patients with 12 to 24 months of follow-up available had observed 100% overall survival through Kaplan Meier estimate at 12 months post-infusion. The data also showed improvement in wound healing skills and remission of a skin rash associated with LAD-I.
- There have been no significant adverse events associated with RP-L201 to date, but all patients had a very positive safety profile for the drug. It has been previously reported that adverse effects connected to other study techniques, such as busulfan conditioning, are consistent with the safety profiles of those agents and techniques.
- Rocket expects to submit the Biologics License Application (BLA) with the FDA in the second quarter of 2023 in light of the promising efficacy and safety data from the Phase 2 pivotal LAD-I study.
Restraints of Egypt Leukocyte Adhesion Deficiency (LAD-I) Therapeutics Market
Because of the high cost of development and the small patient population, companies are likely to encounter difficulties while bringing their medications to market.
Key players
Alexion Pharmaceuticals Sanofi Grifols Kedrion Bioverativ Alnylam Pharmaceuticals Apellis Pharmaceuticals Stealth Biotherapeutics Dyax Shire
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Egypt Leukocyte Adhesion Deficiency (LAD-I) Therapeutics Market
By Treatment
- Hematopoietic Stem Cell Transplantation
- Recombinant Human Interferon-gamma Treatment
- Prophylactic Immunoglobulin Therapy
- Antimicrobial Therapy
- Prophylactic Therapy
- Fucose Supplementation
- Monoclonal Antibodies
- Coagulation Factors
By End Users
- Hospitals
- Specialty Clinics
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































