Egypt Constipation Therapeutics Market Analysis
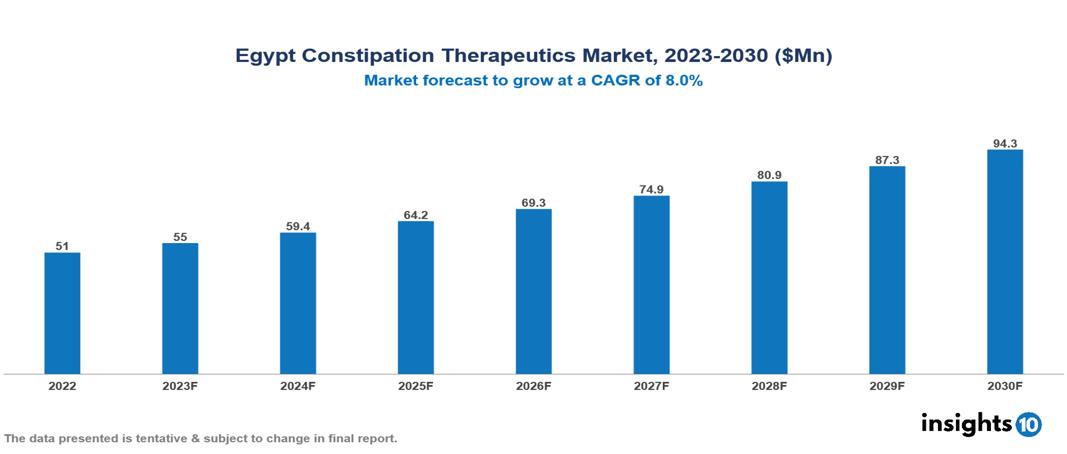
Egypt Constipation Therapeutics Market was valued at $51 Mn in 2022 and is estimated to reach $94 Mn in 2030, exhibiting a CAGR of 8% during the forecast period. The global growth of the constipation therapeutics market is driven by factors such as aging, sedentary lifestyles, and unhealthy dietary habits. The increasing elderly population, susceptible to chronic conditions, notably contributes to this expansion. Major players in this sector comprise Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck & Co., Novartis, and Pfizer.
Buy Now

Egypt Constipation Therapeutics Market Executive Summary
Egypt Constipation Therapeutics Market was valued at $51 Mn in 2022 and is estimated to reach $94 Mn in 2030, exhibiting a CAGR of 8% during the forecast period.
Constipation is a digestive disorder characterized by infrequent, challenging, or hardened bowel movements resulting from the slowed movement of stool through the colon, leading to increased water absorption and solidification of stool. This condition arises from a reduction in the speed at which feces traverse the large intestine. Common symptoms of constipation include exertion during bowel movements, a feeling of incomplete evacuation, abdominal discomfort, and irregular or infrequent bowel patterns. Various factors, including a low-fiber diet, insufficient fluid intake, lack of physical activity, specific medications, and underlying medical conditions, can contribute to the development of constipation. Managing constipation typically involves making lifestyle adjustments, modifying dietary habits, increasing physical activity, and, when necessary, using medications to address and alleviate symptoms.
Constipation is a prevalent gastrointestinal issue in Egypt, impacting individuals across various age groups, with a reported prevalence of 23.3%. A significant portion of the population, approximately 56%, does not consume whole grains daily, contributing to constipation risk. Factors influencing constipation in Egypt encompass dietary habits, lifestyle choices, and potential medical conditions. Traditional diets, characterized by a high intake of refined grains and low fiber content, are implicated in the prevalence of constipation. Sedentary lifestyles and insufficient water intake further contribute to digestive difficulties. Addressing constipation involves lifestyle modifications, including increasing fiber intake through fruits, vegetables, and whole grains, maintaining proper hydration, and incorporating regular physical activity. Additional contributors to constipation include a diet low in fiber, dehydration, lack of physical activity, certain medications, and stress, all of which underscore the importance of comprehensive strategies and healthcare interventions for effective management and prevention.
Representing a groundbreaking approach to address chronic constipation is the recently approved Vibrant capsules, sanctioned by the US Food and Drug Administration in August and now available for prescription. Unveiling an innovative drug-free alternative, these capsules, similar in size to a regular pill, vary from conventional medication delivery methods. Rather than releasing medication post-ingestion, they employ innovative technology by vibrating to stimulate the colon. This pioneering solution offers individuals grappling with chronic constipation a new and potentially effective means to facilitate bowel movements, marking a noteworthy advancement in constipation management.
Market Dynamics
Market Growth Drivers
Rising Geriatric Population: In 2020, around 8% of Egypt's total population, equivalent to approximately 8.4 Mn people, were aged 60 or older. Projections indicate that by 2050, this demographic will more than double, reaching 22 Mn individuals and making up 14% of the population. The aging population in Egypt is anticipated to result in a higher incidence of constipation, a common concern among older individuals. This demographic shift is likely to drive an increased demand for constipation therapeutics tailored to meet the specific needs of the elderly.
Changing Lifestyle and Dietary Patterns: Modernization and lifestyle modifications frequently result in dietary changes, such as a decrease in fiber intake and sedentary behavior, which exacerbate constipation. Constipation may result from a switch to a Westernized diet, which is frequently higher in processed foods and lower in fiber. This in turn increases the need for treatments to deal with and manage problems associated with constipation.
Technological Advancements in Treatment Options: Advances in pharmaceutical research and development, leading to the introduction of more effective and targeted constipation therapeutics, can stimulate market growth. Innovative treatment options with improved efficacy and reduced side effects may gain traction among healthcare providers and patients.
Market Restraints
Economic Constraints: Economic factors, including lower income levels in certain segments of the population, may limit the affordability of constipation therapeutics. This could lead to a reduced adoption rate of medications or treatments, particularly among individuals with financial constraints.
Limited Healthcare Awareness: The lack of awareness or understanding about the importance of gastrointestinal health and the available constipation therapeutics may hinder market growth. Limited awareness among both healthcare professionals and the general population might result in delayed or inadequate treatment seeking.
Cultural and Dietary Practices: Traditional cultural and dietary practices in Egypt, which may not prioritize a high-fiber diet or active lifestyle, could pose challenges for preventive measures against constipation. Cultural preferences and habits may need to be considered in the development and promotion of therapeutics.
Healthcare Policies and Regulatory Landscape
The Ministry of Health and Population (MOHP) serves as the primary regulatory authority in Egypt responsible for overseeing the approval, importation, manufacturing, and distribution of pharmaceuticals, including therapeutic medications. Additionally, the MOHP plays a key role in formulating healthcare policies and conducting regulatory monitoring of these medications. Collaborating closely with the MOHP, the Central Administration for Pharmaceutical Affairs (CAPA) is tasked with regulating drugs, overseeing licensing procedures, and ensuring quality control. The CAPA works in conjunction with the Egyptian Drug Authority (EDA) to actively monitor the effectiveness and safety of pharmaceuticals. Both the MOHP and the EDA have established regulations pertaining to clinical trials for therapeutic medications, and manufacturers are required to adhere to Good Manufacturing Practice (GMP) guidelines to ensure the quality of their products. Furthermore, the MOHP exercises control over pharmaceutical import and export processes, in addition to regulating pharmaceutical prices and reimbursement.
Competitive Landscape
Key Players
- Abbott
- Amgen
- AstraZeneca
- Bayer
- Boehringer Ingelheim
- Eli Lilly
- GlaxoSmithKline
- Merck & Co.
- Novartis
- Pfizer
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Egypt Constipation Therapeutics Market Segmentation
By Therapeutic
- Laxatives
- Chloride Channel Activators
- Peripherally Acting Mu-Opioid Receptor Antagonists
- GC-C Agonists
- 5-HT4 Receptor Agonists
By Disease
- Chronic Idiopathic Constipation
- Irritable Bowel Syndrome with Constipation
- Opioid-Induced Constipation
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.