Egypt Central Nervous System (CNS) Therapeutics Market Analysis
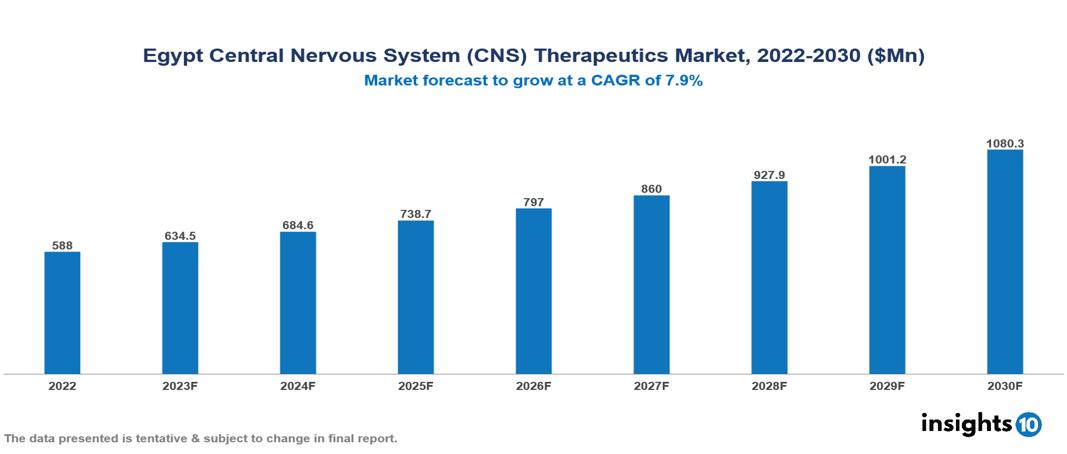
The Egypt Central Nervous System (CNS)Therapeutics Market was valued at $588 Mn in 2022 and is predicted to grow at a CAGR of 7.9% from 2023 to 2030, to $1,080 Mn by 2030. The key drivers of this industry include the upward surge in the prevalence of CNS disorders, high unmet medical needs, and the growing pharmaceutical industry. The industry is primarily dominated by players such as Hikma, Novartis, EIPICO, AstraZeneca, Pharco, Eva, Pfizer, and Roche among others.
Buy Now

Egypt Central Nervous System (CNS) Therapeutics Market Executive Summary
The Egypt Central Nervous System (CNS)Therapeutics Market is at around $588 Mn in 2022 and is projected to reach $1,080 Mn in 2030, exhibiting a CAGR of 7.9% during the forecast period.
Neurological disorders cover a wide range of conditions affecting both the central and peripheral nervous system, which includes the brain, spinal cord, and nerves. These disorders exhibit diverse symptoms such as headaches, limb numbness or weakness, dizziness, cognitive difficulties, speech and vision impairments, and tremors. Examples of common neurological disorders include Alzheimer's disease, epilepsy, multiple sclerosis, and Parkinson's disease. The origins of these disorders are multifaceted, involving factors like genetics, infections, lifestyle-related issues, environmental influences, and underlying health conditions. Treatment approaches for neurological disorders vary and may include medications, physical therapy, occupational therapy, and, in certain cases, surgical interventions. Prominent companies involved in the production, research, and development of therapeutics for neurological disorders include UCB, Eisai, Biogen, Novartis, and Roche, showcasing a dynamic landscape of efforts to address these intricate conditions.
The estimated prevalence of neurological conditions is more than 17% in Egypt. The market therefore is driven by major contributors like a surge in the prevalence of CNS disorders, high unmet medical needs, and the growing pharmaceutical industry. However, affordability challenges, health system disparities, and a complex regulatory landscape limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Increasing prevalence of neurological diseases: Egypt is experiencing an escalating challenge of neurological conditions such as Alzheimer's disease, Parkinson's disease, epilepsy, and depression. The estimated prevalence of CNS disorders including anxiety and others is more than 17%. Various factors contribute to this situation, including an aging population. The increasing life expectancy in Egypt implies a growing number of individuals vulnerable to age-related neurodegenerative diseases. Additionally, lifestyle changes such as rising urbanization, unhealthy dietary habits, and insufficient physical activity further contribute to the heightened risk of developing neurological disorders.
High unmet medical need: The demand for innovative and improved treatment options is fuelled by the existing medical gaps in various neurological disorders. Pharmaceutical companies are proactively developing new therapeutics, such as targeted therapies and personalized medicine approaches, to generate interest in the market. Moreover, the growing utilization of advanced technologies like gene therapy and neurostimulation in addressing CNS disorders provides additional avenues for growth.
Growing pharmaceutical industry: The pharmaceutical sector in Egypt is experiencing a steady expansion, marked by increased investments in domestic manufacturing and research & development (R&D). The rise in the production of generic CNS medications is noteworthy, given their affordability compared to brand-name drugs, thereby making them more accessible to a broader segment of the population.
Market Restraints
Disparities in the health system: Egypt encounters a deficiency in neurologists, psychiatrists, and other specialists with the expertise to diagnose and address CNS conditions. This shortage could result in delayed diagnoses, inappropriate treatment, and less-than-ideal patient outcomes. The uneven distribution of healthcare facilities is also a concern, as specialized healthcare and mental health services may be concentrated in urban areas, leaving individuals in rural regions with restricted access to accurate diagnosis and proper treatment.
Stringent regulatory environment: The approval procedures set by the Egyptian Drug Authority (EDA) are rigorous, emphasizing safety and efficacy in drug approval. Although essential, these processes can be prolonged and complex, resulting in delays in introducing new CNS therapies to the market. The stringent requirements for clinical trials can increase the financial and temporal challenges for pharmaceutical companies, possibly dissuading them from entering the Egyptian market. Moreover, obstacles in securing insurance coverage for new drugs, stemming from limited data or approval delays, can additionally impede the growth of the market.
Affordability challenges: Budgetary limitations in Egypt's healthcare system affect the availability of costly CNS medications, posing a challenge for individuals who have to bear out-of-pocket expenses, especially those dealing with chronic conditions that necessitate prolonged treatment. The absence of universal health coverage specifically for CNS disorders adds to the difficulty of access, particularly for low-income groups. Additionally, inadequate insurance coverage for CNS therapies, particularly innovative or specialized medications, serves as an additional obstacle.
Healthcare Policies and Regulatory Landscape
In Egypt, the main regulatory body overseeing the approval and licensure of drugs and pharmaceuticals is the Egyptian Drug Authority (EDA), which operates under the Ministry of Health and Population. The EDA is responsible for ensuring the safety, efficacy, and quality of pharmaceutical products in the country.
To obtain licensure for drugs, manufacturers must submit a comprehensive application to the EDA. The regulatory process involves a thorough review of these submissions to assess compliance with national and international standards. Once approved, the product receives a marketing authorization, allowing it to be distributed and sold in the Egyptian market.
The regulatory environment for new entrants in the pharmaceutical industry in Egypt can be demanding due to the stringent requirements set by the EDA. New companies seeking to enter the market must navigate a complex regulatory framework, ensuring that their products meet all necessary standards. This includes conducting and submitting extensive pre-clinical and clinical trial data, adhering to Good Manufacturing Practices (GMP), and demonstrating compliance with pharmacovigilance and post-marketing surveillance requirements. While the regulatory process may pose challenges, it ultimately aims to safeguard public health by ensuring that pharmaceutical products meet the necessary quality and safety standards before reaching consumers in Egypt.
Competitive Landscape
Key Players
- Novartis
- Roche
- Pfizer
- Boehringer Ingelheim
- Eva Pharma
- Pharco Pharmaceuticals
- AstraZeneca
- Hikma Pharmaceuticals
- EIPICO
- GSK plc
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Egypt Central Nervous System (CNS) Therapeutics Market Segmentation
By Drug
- Biologics
- Non-Biologics
By Drug Class
- Antidepressants
- Analgesics
- Immunomodulators
- Interferons
- Decarboxylase Inhibitors
- Others
By Disease
- Neurovascular Disease
- Degenerative Disease
- Infectious Disease
- Mental Health
- CNS Cancer
- Others
By Distribution Channel
- Hospital based pharmacies
- Retail pharmacies
- Online pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.