Egypt Blood Disorder Therapeutics Market
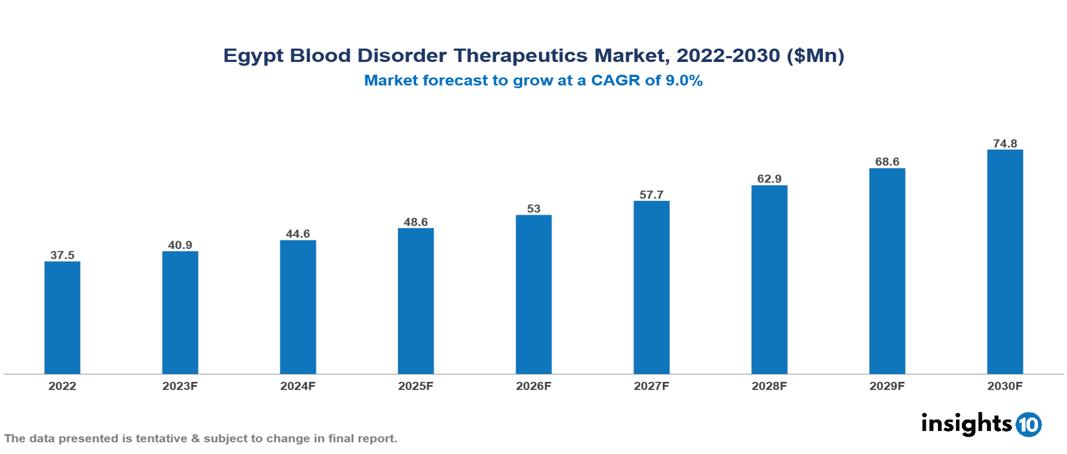
Egypt Blood Disorder Therapeutics Market valued at $38 Mn in 2022, projected to reach $75 Mn by 2030 with a 9% CAGR. The market is fuelled due to a push from a mix of certain factors like the increased prevalence of Blood Disorders in the country, supported by increased awareness and acceptance and availability of treatment options due to recent technological advancements. The Egypt Blood Disorder Therapeutics Market encompasses various players across different segments, including Takeda, Roche, Novartis, Pfizer, AstraZeneca, Sanofi, Grifols, Octapharma, Pharco Pharmaceuticals, EVA Pharmaceuticals etc, among various others.
Buy Now

Egypt Blood Disorder Therapeutics Market Executive Summary
Egypt Blood Disorder Therapeutics Market valued at $38 Mn in 2022, projected to reach $75 Mn by 2030 with a 9% CAGR.
A blood disorder is a medical condition characterized by abnormalities in either white blood cells, responsible for immune responses, red blood cells, which carry oxygen, or platelets, crucial for blood clotting. These disorders can lead to various symptoms, including unexplained fatigue and weight loss, impacting the proper functioning of the blood. Many of these conditions are associated with genetic factors, often resulting from mutations in specific gene regions. The severity of blood disorders varies, and their malignancy depends on the underlying causes. Factors such as certain medical conditions, medications, and lifestyle choices can contribute to their development. Treatment options include pharmacotherapy, such as using drugs like romiplostim to stimulate platelet production, antibiotics for white blood cell disorders, and dietary supplements for anemia caused by deficiencies in iron, vitamin B-9, or B-12. In more severe cases, interventions like blood transfusion therapy, chemotherapy, radiation therapy, immunotherapy, and stem cell transplantation may be considered.
Hemophilia A and B are hereditary bleeding diseases that afflict around one in 10,000 girls and one in 50,000 males. In the Egyptian population, thalassemia is the most frequent cause of chronic hemolytic anemia and is associated with a high rate of morbidity and death.
The market is fuelled due to a push from a mix of certain factors like the increased prevalence of Blood Disorders in the country, supported by increased awareness and acceptance and availability of treatment options due to recent technological advancements.
Takeda is a prominent player in the worldwide hemophilia treatment industry, suggesting that it may also be leading this market in Egypt. Pharco Pharmaceuticals is a well-known Egyptian pharmaceutical business having a sizable stake in certain locally made or marketed medications with its expanding array of therapies for blood disorders.
Market Dynamics
Market Growth Drivers
Increasing prevalence: Hemophilia, thalassemia, and sickle cell disease are among the blood diseases that are becoming more common in Egypt, in line with worldwide trends. Numerous variables, including expanding populations, better diagnosis, and more awareness, might be blamed for this growth. In Egypt, there are about 3,000 people who have hemophilia. This figure, together with the population growth, indicates a sizable patient base that is fueling the need for treatments.
Increasing awareness and enhancing diagnosis: In Egypt, more government initiatives and public awareness campaigns are fostering better knowledge and early detection of blood problems. This makes it possible for prompt management and intervention, which raises the need for available treatment choices. The market for therapies is ultimately driven by initiatives like the National Hemophilia Program and Thalassemia Control Program, which increase awareness and improve access to diagnostics.
Treatment and technological developments: Gene therapy and innovative drug delivery methods are two examples of the ongoing treatment options advancements that the global blood disease therapeutics market is seeing. Egypt is gradually gaining access to these developments, which provide optimism for improved patient outcomes and draw capital to the sector.
Market Restraints
Stigma and cultural opinions: Some cultural attitudes about blood diseases have the potential to generate stigma and deter families from getting a diagnosis or receiving treatment. Improving patient outcomes requires dispelling these myths and increasing knowledge. Healthcare professionals must comprehend the potential connections between the use of conventional therapies and traditional medicine practices.
Inadequate healthcare infrastructure: Differences in access to diagnosis and treatment arise from the uneven dispersion of healthcare facilities between urban and rural locations. Patients who live in rural areas may find it difficult to go to specialized care facilities as a result of this. Diagnostic and treatment capacity is further limited by a shortage of qualified healthcare workers, particularly hematologists, and specialists in certain blood diseases.
Excessive treatment costs: Patients and their families are frequently burdened by the exorbitant expense of imported blood disease medications. Access to necessary therapies is hampered by this restricted cost, especially for people without insurance or sufficient service coverage. Manufacturing generic medication locally can be a more affordable option, but building strong manufacturing skills is a difficulty.
Healthcare Policies and Regulatory Landscape
The Egyptian Drug Authority (EDA) is crucial for upholding public health standards by overseeing the regulation of pharmaceuticals and healthcare products in Egypt. Operating under the Ministry of Health and Population, the EDA is responsible for ensuring that drugs available in the country meet rigorous criteria for safety, efficacy, and quality. Its duties span the entire lifecycle of pharmaceutical products, including approval, registration, and ongoing post-market surveillance. A key role of the EDA is to assess and grant marketing authorization to drugs, subjecting them to a comprehensive evaluation of scientific and clinical data to establish their safety and effectiveness before they are permitted to enter the Egyptian market. Following approval, the EDA remains vigilant in monitoring drug safety through activities related to pharmacovigilance. This involves the continuous collection, evaluation, and response to information about adverse effects or any issues related to drugs. The EDA collaborates with healthcare professionals, manufacturers, and the public to maintain a comprehensive and timely reporting system. Furthermore, the EDA engages in international collaborations and adheres to global regulatory standards, staying informed about developments in pharmaceutical science and regulation. Through the enforcement of stringent regulatory measures, the EDA significantly contributes to upholding the quality and safety of pharmaceuticals in Egypt, thereby fostering public confidence in the healthcare system.
Competitive Landscape
Key Players:
- Takeda
- Roche
- Novartis
- Pfizer
- AstraZeneca
- Sanofi
- Grifols
- Octapharma
- Pharco Pharmaceuticals
- EVA Pharmaceuticals
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Egypt Blood Disorder Therapeutics Market Segmentation
By Disorder:
- Anemia
- Hemophilia
- Leukemia
- Myeloma
- Lymphoma
- Rare blood disorders
By Product Type
- Plasma-derived therapeutics
- Recombinant therapeutics
- Gene therapy
- Other therapies
By End User
- Hospitals
- Specialty clinics
- Ambulatory care
- Home healthcare
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.