Egypt Biosensors Market Analysis
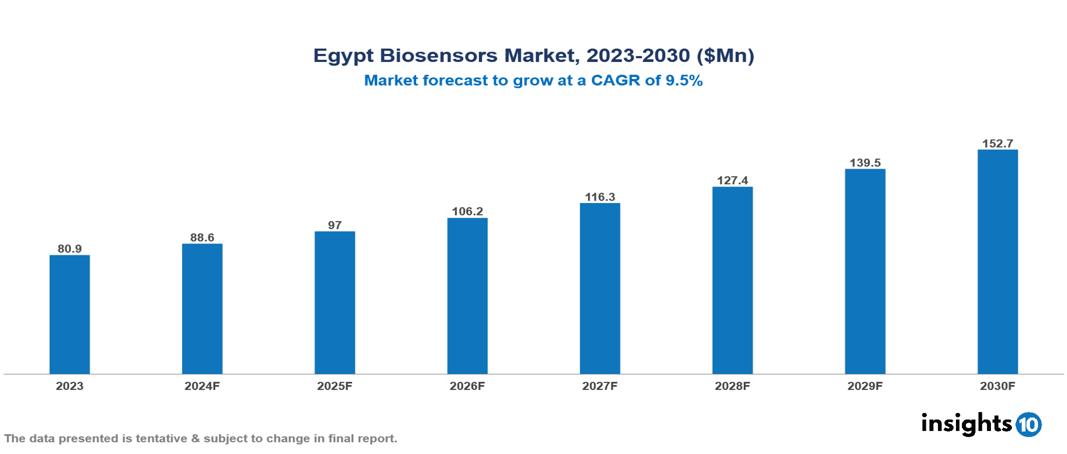
The Egypt Biosensors Market was valued at $80.9 Mn in 2023 and is predicted to grow at a CAGR of 9.5% from 2023 to 2030, to $152.7 Mn by 2030. The key drivers of the market include increasing burden of chronic diseases, aging population, and growing demand for Point-of-Care (POC) testing. The prominent players of the Egypt Biosensors Market are BM-Egypt, Kimal, Medtronic, Abbot Laboratories, and Johnson & Johnson, among others
Buy Now

Egypt Biosensors Market Executive Summary
The Egypt Biosensors market is at around $80.9 Mn in 2023 and is projected to reach $152.7 Mn in 2030, exhibiting a CAGR of 9.5% during the forecast period.
Biosensors are devices that convert a biological response into a measurable electrical signal. They generate signals which are proportional to the concentration of the analyte in the reaction. Applications of biosensors include disease monitoring, drug discovery and development, and the identification of contaminants, pathogen-causing microorganisms, and disease-indicating markers in physiological fluids such as blood, urine, saliva, and sweat. A biosensor consists of components, namely the analyte, bioreceptor, transducer, electronics, and the display. Analyte is the target molecule which is to be detected in a sample. For instance, an analyte could be glucose which is to be measured in a diabetic patient. A bioreceptor is molecule that specifically recognises the analyte. Examples of bioreceptors include enzymes, cells, aptamers, DNA, and antibodies. Bio-recognition is the process of generating a signal in the form light, heat, pH, charge or mass shift when the bioreceptor and analyte interact. Another important component of biosensors is the transducer which functions to convert one form of energy into another, more specifically it converts the biological signal from the bioreceptor into an electrical signal in a process known as signalisation. Transducers can be electrochemical, optical, or piezoelectric. Electronics is the part of a biosensor that processes the transduced signal and prepares it for display. Lastly, the display consists of a user interpretation system such as the LCD of a computer or a direct printer which generates an output based on the requirements of the end user.
Chronic diseases, particularly Noncommunicable diseases (NCDs), including cardiovascular diseases, diabetes, cancer, and chronic respiratory diseases, are currently the leading national causes of death in Egypt. The Egypt Biosensors Market is thus driven by significant factors such as the increasing burden of chronic diseases, aging population, and growing demand for Point-of-Care (POC) testing. However, high costs and limited reimbursement, technical challenges, and data management issues restrict the growth and potential of the market.
The leading players of the Egypt Biosensors Market are BM-Egypt, Kimal, Medtronic, Abbot Laboratories, and Johnson & Johnson, among others.
Market Dynamics
Market Growth Drivers
Increasing Burden of Chronic Diseases: These are a major public health concern, placing a significant strain on the healthcare system and individual well-being. NCDs are estimated to account for 82% of all deaths and 67% of premature deaths. As these diseases become more prevalent, ongoing evaluation of health parameters is crucial for effective management. Biosensors provide a convenient, non-invasive method for patients to monitor vital health metrics at home, decreasing the necessity for frequent hospital visits. Biosensors also allows the patients to resume their daily working routines and lead to higher quality of life. Real-time monitoring with biosensors allows for better chronic disease management and timely treatment, preventing complications and enhancing long-term health outcomes, which is propelling the biosensors market's growth.
Aging Population: Egypt is going through a demographic transition as the number of persons aged 60+ is expected to more than double between 2020-2050 from 8.4 Mn (8% of the total population) to 22 Mn (14%). Through the use of biosensors, the old aged people can use these biosensors as monitors for the chronic conditions without requiring frequent doctor visits. Overall, the aging population increases the demand for biosensors due to the advantages it offers, which contributes to the growth of the Biosensors Market.
Growing Demand for Point-Of-Care (POC) Testing: The growing demand for Point-Of-Care (POC) testing significantly drives the growth of the biosensors market for various reasons. Biosensors are particularly suited for POC testing due to their portability, ease of use, and ability to deliver rapid results—benefits that traditional lab-based testing methods often lack. Additionally, POC testing with biosensors enhances healthcare accessibility, even in remote areas or for patients with limited mobility. The COVID-19 pandemic demonstrated the crucial role of POC testing, with biosensors being instrumental in managing the crisis. As a result, the increasing demand for POC testing is accelerating the growth of the biosensors market.
Market Restraints
High Costs and Limited Reimbursement: Manufacturing biosensors can be expensive, potentially stifling innovation and resulting in high initial costs for the equipment to offset production expenses. If biosensors are not economically competitive with traditional methods, healthcare providers may hesitate to adopt and recommend them. Additionally, insurance companies may not fully reimburse these costs, limiting patients’ access to biosensor technologies. Consequently, the high costs and restricted affordability of biosensors can hinder the growth of the biosensors market.
Technical Challenges: The continuous progress in biosensors is transforming healthcare, yet certain technical challenges persist. To ensure precise diagnoses, biosensor sensitivity, specificity, and accuracy need constant improvement. One major issue is the ability of biosensors to differentiate the target analyte from other sample constituents. Furthermore, detecting low analyte concentrations is critical for early disease detection and environmental monitoring. Reproducibility of biosensor results is also essential but challenging. Overcoming these issues is necessary but slows the growth of the biosensors market.
Data Management Issues: Biosensors create a significant amount of health data that needs to be efficiently stored, organized, and analysed, requiring a robust data management infrastructure and advanced solutions. This can be costly and challenging to manage. Accurate and precise data is essential for effective analysis and decision-making, especially in the medical field. Integrating data from multiple biosensors is also challenging due to different formats, standards, and protocols, complicating data sharing and interoperability across departments. Consequently, these data management issues can impede the growth of the biosensors market.
Regulatory Landscape and Reimbursement Scenario
The Egyptian Drug Authority (EDA) is Egypt’s pharmaceutical regulatory body, overseen by the Ministry of Health. It plays a vital role in ensuring the efficacy, safety, and quality of medications that are made available in the nation. The comprehensive drug approval procedure that the EDA enforces helps guarantee that only safe and effective pharmaceuticals are supplied to the Egyptian market; innovative medications that have undergone adequate testing are beneficial to patients; and public health is protected by reducing the risks associated with potentially harmful or ineffective medications.
Before new pharmaceuticals are offered in Egypt, the EDA examines applications and authorizes their marketing. It also grants licenses to producers, distributors, and wholesalers of pharmaceuticals and medical equipment. The EDA ensures medications fulfil the criteria of quality necessary for the purposes for which they are intended. This entails conducting quality control procedures, keeping up a national drug control laboratory, and inspecting manufacturing sites. When evaluating novel drugs, the EDA examines and approves clinical trial applications to guarantee participant safety and ethical research procedures. After a drug is placed in the market, the EDA actively monitors its safety by gathering and evaluating information on side effects that patients and medical experts have reported.
Egypt’s health insurance landscape includes the Universal Health Insurance (UHI) program. The UHI Law was passed in 2017 and replaced the previously fragmented insurance system of Egypt. The law aimed to provide comprehensive health insurance coverage to all Egyptian citizens. The goal of the UHI program is to provide a uniform benefits package that includes services for preventive care (checkups, screenings), services for both inpatient and outpatient care, necessary prescription drugs, maternity care, and emergency medical attention.
Competitive Landscape
Key Players
Here are some of the major key players in the Egypt Biosensors Market:
- BM-Egypt
- Kimal
- Medtronic
- Abbot Laboratories
- Johnson & Johnson
- Bio-Rad International
- Biosensors International
- Siemens Healthcare
- F. Hoffmann- La Roche
- Meridian Bioscience
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Egypt Biosensors Market Segmentation
By Technology
- Electrochemical Biosensors
- Optical Biosensors
- Piezoelectric Biosensors
- Thermal Biosensors
- Nanomechanical Biosensors
By Product
- Wearable Biosensors
- Non-wearable Biosensors
By Application
- Medical Diagnostics
- Food Safety
- Environmental Monitoring
- Agriculture and Bioreactor Monitoring
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































