Egypt Biomaterials in Healthcare Market Analysis
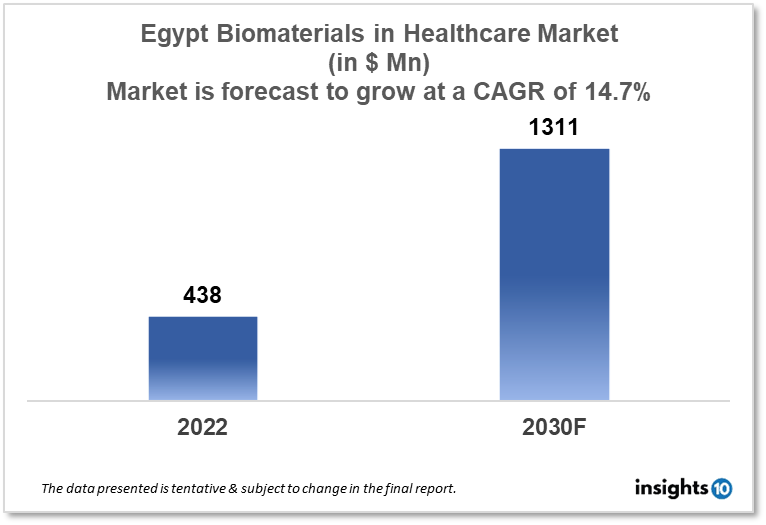
The Egypt Biomaterials in Healthcare Market is expected to witness growth from $438 Mn in 2022 to $1311 Mn in 2030 with a CAGR of 14.70% for the forecasted year 2022-2030. As Egypt's population ages, there is a growing demand for biomaterials to treat conditions like osteoarthritis, spine issues, and cardiovascular disease. The market is segmented by type and by application. Some key players in this market include: MedNotch, Procare, BASF SE, Johnson and Johnson, Medtronic and Evonik Industries.
Buy Now

Egypt Biomaterials in Healthcare Market Executive Analysis
The Egypt Biomaterials in Healthcare Market size is at around $438 Mn in 2022 and is projected to reach $1311 Mn in 2030, exhibiting a CAGR of 14.70% during the forecast period. Only 5.2% of Egypt's overall budget, or 1.5% of GDP, was allotted to the health sector up until 2021. From 4.9% in 2019 to 4.2% in 2022, the budget's share of health expenses decreased, reaching its lowest level in the preceding five years. At 1.6% of GDP, government spending on health reached a record high. In Egypt, public health spending has grown to 3% of GDP.
Biomaterials are elements that are applied in medical settings to repair, enhance, or sustain organs or tissues that are diseased or damaged. They are increasingly being used in Egypt's healthcare market for a variety of purposes. Biomaterials can be used to build scaffolds that replicate the natural environment of cells, enabling them to grow and differentiate into new tissues. Dental implants, cardiovascular devices, joint replacements, and other types of implants can all be made of biomaterials. Biomaterials can be used to encapsulate and transport medications to particular parts of the body, enhancing their efficacy and minimising their side effects. Dressings and other items that aid in the healing of burns and other lesions can be made using biomaterials. Biomaterials are made to work with the body, lowering the possibility of rejection and other negative responses. The effectiveness and danger of complications of biomaterials can be enhanced by customising them for particular patients or applications. There is less of a need for regular replacements because many biomaterials are made to last for years or even decades. By enhancing outcomes and lowering the need for additional treatments, the use of biomaterials can frequently lower healthcare expenses.
Market Dynamics
Market Growth Drivers
As Egypt's population ages, there is a growing demand for biomaterials to treat conditions like osteoarthritis, spine issues, and cardiovascular disease. The growth of the biomaterials industry is a priority for the Egyptian government. This includes funding for R&D, tax breaks, and regulatory support—all of which help the Egyptian biomaterial healthcare market improve and expand. In Egypt, conditions like cancer, diabetes, and cardiovascular diseases are becoming more common. Biomaterials that can be used in the therapy and management of these conditions are now required as a result of this. which causes the market for medicinal biomaterials in Egypt to grow. The Egyptian biomaterial healthcare market now offers new opportunities for manufacturers because of advances in biomaterials technology. For instance, the development of biodegradable materials has products that the body can securely absorb.
Market Restraints
In Egypt, Manufacturers might have trouble getting the funding they need to expand their businesses, invest in R&D, and engage in competitive bidding for the Egypt biomaterial healthcare market. It can be challenging to navigate Egypt's regulatory environment due to its complexity. The approvals and licences that manufacturers need to sell their goods in Egypt's biomaterial healthcare market might be difficult to obtain. The Egypt Biomaterials in Healthcare market is experiencing a skills gap, which can make it challenging for manufacturers to hire and retain skilled workers.
Competitive Landscape
Key Players
- MedNotch (EG)
- Procare (EG)
- BASF SE
- Johnson and Johnson
- Medtronic
- Evonik Industries
Notable Recent Deals
2021: Probiotic, a regional biotech business specialising in the creation of biomaterials, has a 97.5% ownership stake now owned by Egypt's Minapharm Pharmaceuticals. This $23.5 million deal was intended to improve Minapharm's standing in the expanding biomaterials market in Egypt.
Healthcare Policies and Regulatory Landscape
The Ministry of Health and Population (MoHP) and the Egyptian Drug Authority (EDA) are in charge of Egypt's medical policies and regulatory framework in the Biomaterials in Healthcare industry. The EDA is in charge of regulating and approving biomaterials for use in Egypt, whereas the MoHP is in charge of creating healthcare policies and laws. Biomaterials are categorised as medical devices in Egypt and are subject to EDA regulatory clearance. A biomaterial must go through a regulatory evaluation procedure to make sure it complies with safety and efficacy requirements before it can be marketed and sold in Egypt. Guidelines for the registration of medical devices have been set forth by the EDA, and they include specifications for product testing, clinical trials, labelling, and paperwork. Biomaterials are subject to import restrictions in Egypt in addition to regulatory approval. Before being brought into Egypt, all imported medical devices, including biomaterials, must be registered with the EDA, according to the Egyptian government. An evaluation of the product's efficacy and safety, as well as its compliance with Egyptian laws, are all part of the registration process.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Biomaterials in Healthcare Market Segmentation
By Type (Revenue, USD Billion):
Based on type, the market is segmented into Metallic Biomaterials, Polymeric Biomaterials, Ceramic Biomaterials, and Natural Biomaterials. The Metallic Biomaterials segment accounted for the largest share of the Egypt market in 2019. The growing geriatric population Egyptly is expected to drive growth for this segment.
- Metallic Biomaterials
- Stainless Steel
- Titanium & Titanium Alloys
- Cobalt-Chrome Alloys
- Gold
- Silver
- Magnesium
- Polymeric Biomaterials
- Polymethylmethacrylate
- Polyethylene
- Polyester
- Silicone Rubber
- Nylon
- Polyetheretherketone
- Other Polymeric Biomaterials
- Ceramics
- Calcium Phosphate
- Zirconia
- Aluminum Oxide
- Calcium Sulfate
- Carbon
- Glass
- Natural Biomaterials
- Hyaluronic Acid
- Collagen
- Gelatin
- Fibrin
- Cellulose
- Chitin
- Alginates
- Silk
By Application (Revenue, USD Billion):
The cardiovascular, orthopaedic, dental, plastic surgery, wound healing, tissue engineering, ophthalmology, neurological/CNS, and other applications segments are made up of the biomaterials market. The market category for wound healing is anticipated to have the highest CAGR in 2019. The market will increase as a result of factors including expanding healthcare infrastructure, a large population pool, a rising diabetic population, and rising healthcare spending. Surgical Guides
- Cardiovascular
- Catheters
- Stents
- Implantable Cardiac Defibrillators
- Pacemakers
- Sensors
- Heart Valves
- Vascular Grafts
- Guidewires
- Others
- Orthopedic
- Joint Replacement
- Knee Replacement
- Hip Replacement
- Shoulder Replacement
- Others
- Viscosupplementation
- Bioresorbable Tissue Fixation
- Spine?
- Spinal Fusion Surgeries
- Minimally Invasive Fusion Surgeries
- Motion Preservation & Dynamic Stabilization Surgeries
- Pedicle-Based Rod Systems
- Interspinous Spacers
- Artificial Discs
- Fracture Fixation Devices
- Bone Plates
- Screws
- Pins
- Rods
- Wires
- Synthetics Bone Grafts
- Joint Replacement
- Ophthalmology
- Contact Lenses
- Intraocular Lenses
- Functional Replacement of Ocular Tissues
- Synthetic Corneas
- Others
- Contact Lenses
- Dental
- Dental Implants
- Dental Bone Grafts & Substitutes
- Dental Membranes
- Tissue Regeneration
- Plastic Surgery
- Soft-Tissue Fillers
- Craniofacial Surgery
- Wound Healing
- Wound Closure Devices
- Sutures
- Staples
- Surgical Hemostats
- Internal Tissue Sealants
- Adhesion Barriers
- Hernia Meshes
- Wound Closure Devices
- Tissue Engineering
- Scaffolds for Regenerative Medicine
- Nanomaterials for Biosensing
- Tailoring of Inorganic Nanoparticles
?
- Neurological/Central Nervous System Applications
- Shunting Systems
- Cortical Neural Prosthetics
- Hydrogel Scaffolds for CNS Repair
- Neural Stem Cell Encapsulation
?
- Other Applications
- Drug Delivery Systems
- Gastrointestinal Applications
- Bariatric Surgery
- Urinary Applications
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































