Egypt ADHD (Attention Deficit Hyperactivity Disorder) Therapeutic Market Analysis
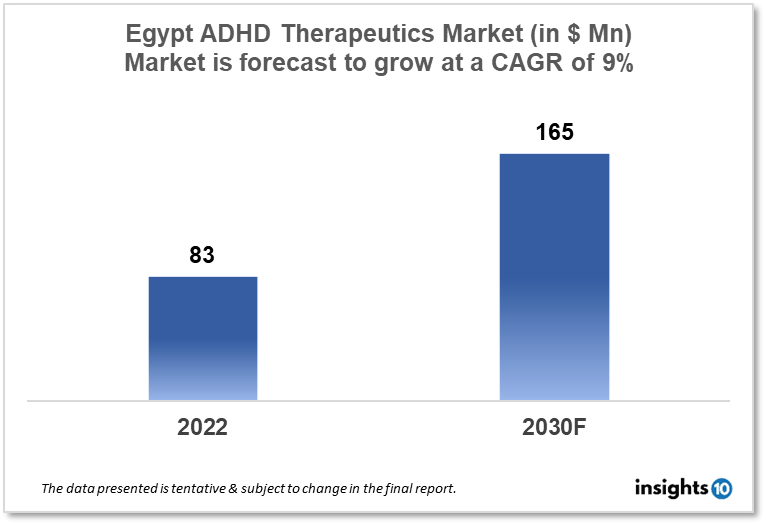
Egypt's Attention Deficit Hyperactivity Disorder (ADHD) therapeutics market is expected to witness growth from $83 Mn in 2022 to $165 Mn in 2030 with a CAGR of 9% for the year 2022-30. The market is projected to expand during the forecast period as the incidence of ADHD increases in Egypt. The Egypt ADHD therapeutics market is segmented by drug, drug type, demographics, and by distribution channel. Clavita Pharma, Zeta Pharma, and Takeda are the major competitors in the market.
Buy Now

Egypt Attention Deficit Hyperactivity Disorder (ADHD) Therapeutics Market Executive Analysis
The Egypt Attention Deficit Hyperactivity Disorder (ADHD) therapeutics market size is at around $83 Mn in 2022 and is projected to reach $165 Mn in 2030, exhibiting a CAGR of 9% during the forecast period. Following the budgetary allotments for public services, social protection, and education, the healthcare investment budget is presently the fourth largest in Egypt's overall public budget. From FY2016-21, healthcare services accounted for an average of 5.2% of total government expenditure, or 1.5% of GDP. A nearly 30% increase from the $0.86 Bn that was allocated for FY 2021–22, public investments in healthcare are scheduled at $1.11 Bn for FY 2022–23. Egypt's government spending is significantly below the global average of 10% and below the MENA average of 6.4%, according to World Bank statistics. Furthermore, it is lower than in Morocco and Tunisia, whose per capita incomes are similar but whose GDP expenditures on healthcare range from 6 to 7 %.
Attention deficit hyperactivity disorder (ADHD) is the most prevalent neurodevelopmental disorder that begins in childhood. The disorder is more common in males than in females. Deficits in focus, activity level, and impulse control are its defining characteristics. According to epidemiological studies, 6.7 to 7.8% of children globally suffer from ADHD, making it a common disorder. In Egypt, this incidence is greater, at 9.4–21.8%. The burden of childhood ADHD raises the likelihood of parenting problems, which are usually linked to issues with child opposition/behavior. In other words, a child's high level of impulsivity or inattentiveness places multiple demands on parents and raises the possibility that they will reply with inconsistent or excessively reactive discipline.
Treatment for ADHD in Egypt frequently entails a combination of medication and behavioral counseling. Psychostimulants like methylphenidate (Ritalin) and atomoxetine (Strattera) are the most frequently given medications for ADHD in Egypt. These drugs function by elevating dopamine and norepinephrine levels in the brain, which aids in enhancing focus and lowering hyperactivity and impulsivity. Cognitive behavioral therapy (CBT) aids those with ADHD in recognizing and altering unfavorable patterns of thought and conduct that contribute to their symptoms. Parent Management Training (PMT) involves teaching parents strategies for managing their child's behavior, such as setting clear expectations and providing positive reinforcement for good behavior.
Market Dynamics
Market Growth Drivers
More people are receiving diagnoses for ADHD as a result of improved diagnostic instruments and greater awareness of the condition. As a consequence, there will probably be a rise in the demand for the Egypt ADHD therapeutics market. The demand for ADHD medication may rise as Egypt's middle class expands because households will have more money to spend on healthcare.
Market Restraints
In Egypt, there is still a lack of knowledge and comprehension of ADHD, which can result in improper diagnosis and care for the condition. Even though access to healthcare is expanding in Egypt, there are still substantial obstacles in some regions, especially in rural and outlying areas. For some people and families in Egypt, especially those with limited financial means, the cost of ADHD treatment, including medication and therapy, can be a barrier.
Competitive Landscape
Key Players
- Liptis (EGY)
- Linkopharm (EGY)
- Bnroshd (EGY)
- Clavita Pharma (EGY)
- Zeta Pharma (EGY)
- Takeda
- Johnson & Johnson
- Novartis
- Eli Lilly
- Tris Pharma
- Neos Therapeutics
Healthcare Policies and Regulatory Landscape
The Egyptian Drug Authority (EDA) is the regulatory body responsible for the approval, registration, and monitoring of pharmaceutical products in Egypt. The EDA was established in 1960 and operates under the jurisdiction of the Egyptian Ministry of Health and Population. Applications for the registration of pharmaceutical goods in Egypt are reviewed and approved by the EDA. Every product must adhere to the authority's strict safety, efficacy, and quality requirements. All pharmaceutical products sold in Egypt must adhere to set quality standards, according to the EDA. This entails regularly checking and testing pharmaceutical goods to make sure they're secure and efficient. The EDA is in charge of issuing licenses to pharmaceutical product distributors and manufacturers in Egypt to make sure they adhere to set safety and quality standards.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
ADHD (Attention Deficit Hyperactivity Disorder) Therapeutic Market Segmentation
By Drug Type (Revenue, USD Billion):
- Stimulants
- Amphetamine
- Methylphenidate
- Dextroamphetamine
- Dexmethylphenidate
- Lisdexamfetamine
- Others
- Non-Stimulants
- Atomoxetine
- Bupropion
- Guanfacine
- Clonidine
By Age Group (Revenue, USD Billion):
- Pediatric And Adolescent
- Adult
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Speciality Clinics
- Retail Pharmacies
- e-Commerce
By Psychotherapy (Revenue, USD Billion):
- Behaviour Therapy
- Cognitive Behavioral Therapy
- Interpersonal Psychotherapy
- Family Therapy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































