China X-Linked Myotubular Myopathy (XLMTM) Drugs Market Analysis
China X-Linked Myotubular Myopathy (XLMTM) Drugs market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for X-Linked Myotubular Myopathy (XLMTM) Drugs is growing rapidly as a result of unmet medical need for therapies of X-Linked Myotubular Myopathy (XLMTM) disease, advancements in gene therapy, Growing Awareness and Patient Advocacy about the disease, Increased Research and Collaboration between research organizations and pharmaceutical companies, supportive regulatory environments, Technological Advancements in gene sequencing and biomarker identification. Novartis Gene Therapies, Avrobio, Axovant Gene Therapies, Sarepta Therapeutics, Amicus Therapeutics, Sanofi Genzyme, Pfizer, Audentes Therapeutics are the key market players operating in global X-Linked Myotubular Myopathy (XLMTM) Drugs market.
Buy Now

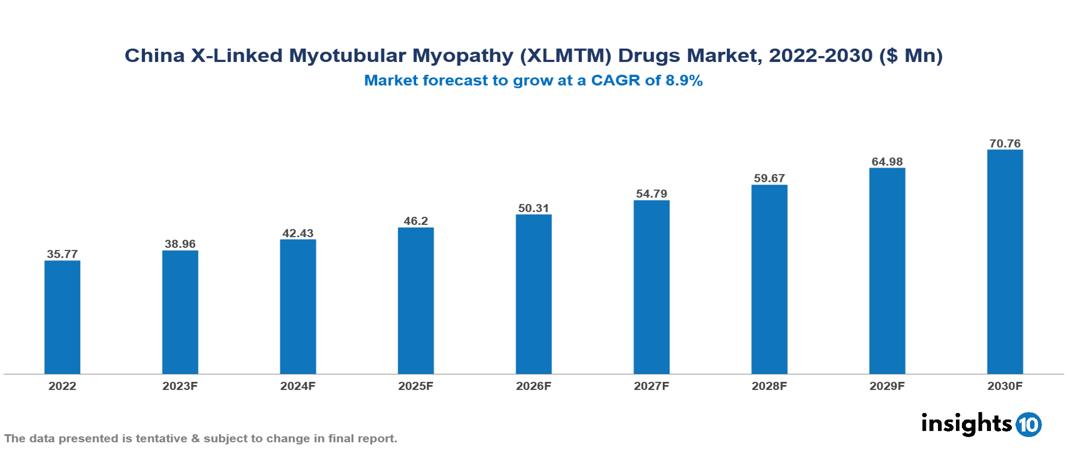
China X-Linked Myotubular Myopathy (XLMTM) Drugs Market Analysis Summary
China X-Linked Myotubular Myopathy (XLMTM) Drugs Market is valued at around $35.77 Mn in 2022 and is projected to reach $70.76 Mn by 2030, exhibiting a CAGR of 8.9% during the forecast period 2023-2030.
The rare genetic condition known as X-Linked Myotubular Myopathy (XLMTM), often referred to as Myotubular Myopathy or X-Linked Centronuclear Myopathy, mostly affects the skeletal muscles. It is brought on by changes in the X chromosome's MTM1 gene. Since males have one X and one Y chromosome, XLMTM mostly affects them. Due to the normal copy of the MTM1 gene on their second X chromosome, females who generally carry the MTM1 mutation are frequently asymptomatic or exhibit lesser symptoms. Common symptoms of this disease are muscle weakness, respiratory complications and reduced lung capacity, delay in motor development like sitting, standing and walking, facial weakness and difficulty in swallowing. Treatment options including supportive care, respiratory support, and potential gene therapies may be considered based on the individual's specific needs. There are no specific drugs approved by regulatory authorities for the treatment of X-Linked Myotubular Myopathy (XLMTM). Management and symptomatic treatment is done.
Market Dynamics
Market Drivers
The unmet medical need for therapies for X-Linked Myotubular Myopathy (XLMTM) disease, advancements in gene therapy, Growing Awareness and Patient Advocacy about the disease, Increased Research and Collaboration between research organizations and pharmaceutical companies, supportive regulatory environments, Technological Advancements in gene sequencing and biomarker identification. All these act as market growth drivers for the development of XLMTM drugs.
Market Restraints
Limited Patient Population due to which there is difficulty in conducting clinical trials and gathering sufficient data.
Complex Disease Biology understanding of the underlying disease mechanisms and molecular pathways is still evolving
High Development Costs in Research and Development of Drugs for X-Linked Myotubular Myopathy (XLMTM) Disease. All these act as market growth restraints.
Key players
Sarepta Therapeutics Inc. Solid Biosciences Inc. Astellas Pharma Prometheus Therapeutics Inc. Sangamo Therapeutics Inc. Alnylam Pharmaceuticals Inc. Audentes Therapeutics Inc. Ultragenyx Pharmaceutical Inc. Shire PTC Therapeutics Inc.1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For China X-Linked Myotubular Myopathy (XLMTM) Drugs market
By Treatment Type
- Gene therapy
- Pharmacological therapy
By Patient Age Group
- Infants
- Children
- Adults
By End-Users
- Hospitals
- Homecare
- Speciality oncology centres
- Others
By Supportive Care
- Respiratory support
- Physical therapy
- Genetic counselling
- Psychological support
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































