China Medication Access Programs Market Analysis
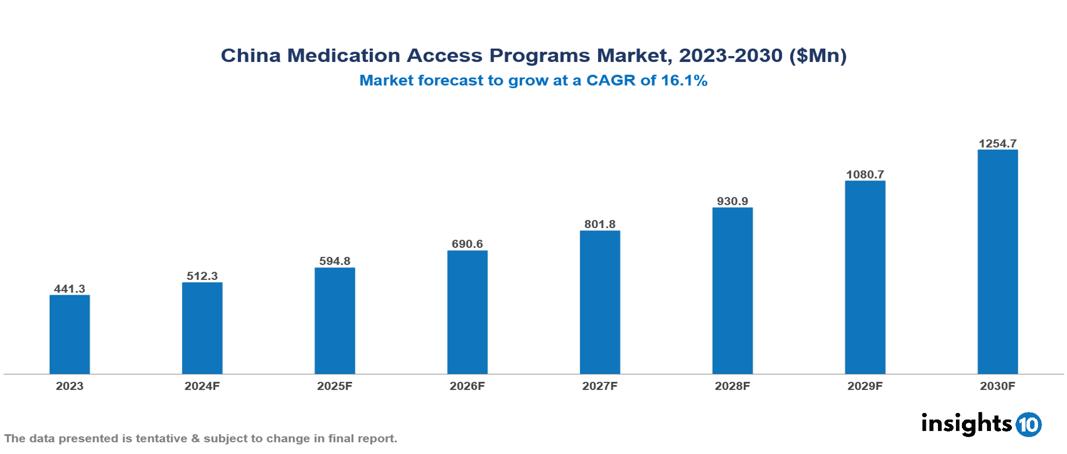
The China Medication Access Programs Market was valued at $441.3 Mn in 2023 and is predicted to grow at a CAGR of 16.1% from 2023 to 2030, to $1254.7 Mn by 2030. The key drivers of this industry include rising healthcare spending, growing chronic disease burden, and government initiatives. The industry is primarily dominated by players such as Gilead Sciences, Takeda Pharmaceuticals, Pfizer, and Novartis among others.
Buy Now

China Medication Access Programs Market Executive Summary
The China Medication Access Programs Market was valued at $441.3 Mn in 2023 and is predicted to grow at a CAGR of 16.1% from 2023 to 2030, to $1254.7 Mn by 2030.
Patient Support Programs (PSPs) are initiatives organized by pharmaceutical companies to enhance access, usage, and adherence to prescription drugs. These programs encompass financial assistance, clinical support, educational efforts, or a blend of these elements. A Medication Access Program (MAP) is a part of PSP which is a crucial connection between patients and their needed medications. Managed Access Programs are initiatives that enable patients with serious or life-threatening illnesses to obtain investigational medicines or treatments not yet commercially available. These programs aim to provide early access to therapies for patients who have exhausted all other options and are ineligible for clinical trials. Offered by pharmaceutical companies, MAPs help patients overcome financial barriers to access essential medications. A centralized, pharmacy-driven MAP can enhance patient outcomes, reduce unnecessary healthcare costs, improve patient and provider satisfaction, streamline patient flow, and boost revenue through increased prescription capture.
In 2021, China's healthcare spending totaled $1.2 Tn, equivalent to 6.5% of its GDP. A study indicated that 99.1% of Chinese adults engage in self-medication. The market is driven by significant factors like rising healthcare spending, growing chronic disease burden, and government initiatives. However, distribution challenges, patient education and compliance, and administrative burdens restrict the growth and potential of the market.
Prominent players in this field include Gilead Sciences and Takeda Pharmaceuticals which provide Medication Access or Access to Medicine Program. Pfizer, Novartis, Merck, and AstraZeneca among others are some of the pharmaceutical companies providing patient support programs and are potential players for the Medication Access Program in China.
Market Dynamics
Market Growth Drivers
Rising Healthcare Spending: In 2021, China allocated $1.2 Tn to healthcare, amounting to 6.5% of its GDP. This substantial investment serves as a significant market driver for medication access programs in China, indicating robust financial support and a growing priority on enhancing healthcare infrastructure, including initiatives to improve access to medications for the population.
Growing Chronic Disease Burden: The widespread occurrence of chronic diseases among Chinese older adults, affecting 81.1% and comprising 179.9 Mn individuals, serves as a crucial market driver for medication access programs in China. This demographic's substantial healthcare needs underscore the demand for effective and accessible treatments, driving the development and expansion of programs that ensure consistent access to essential medications for managing chronic conditions.
Government Initiatives: Government policies promoting healthcare access and affordability via subsidization and reimbursement schemes are key drivers for China's medication access program market. These initiatives foster a conducive environment for expanding programs that enhance medication accessibility, driving demand among healthcare providers and patients.
Market Restraints
Distribution Challenges: Logistical and infrastructure constraints pose a significant market restraint for medication access programs in China by impeding the prompt and efficient distribution of medications to patients across varied geographical areas. These challenges can hinder the effective implementation of access programs, impacting their ability to reliably reach and serve the population in need of medications.
Patient Education and Compliance: A lack of awareness and understanding among patients regarding access programs, combined with issues related to compliance, presents a market restraint for medication access programs in China. This constraint can hinder the effectiveness of these programs by reducing patient participation and adherence, thereby limiting their ability to successfully reach and benefit those in need of essential medications.
Administrative Burden: The administrative complexities of enrolling and managing patients in Medication Access Programs (MAPs) represent a market restraint in China due to their potential to delay enrollment and increase operational burdens for providers and patients alike, hindering program efficiency and scalability.
Regulatory Landscape and Reimbursement Scenario
The National Medical Products Administration (NMPA), formerly known as the China Food and Drug Administration (CFDA), regulates drugs, medical devices, and cosmetics in China. It oversees the evaluation, approval, and ongoing monitoring of these products to ensure safety and efficacy before they can be marketed. Managed access programs (MAPs) provide early patient access to innovative therapies in China, even before formal NMPA approval.
China's health insurance program includes mandatory personal accounts funded by both employers and employees, covering basic outpatient care. Additionally, employers contribute to a pooled fund for inpatient, critical illness outpatient, and chronic disease-related costs. Drug reimbursement in China is managed through the National Reimbursement Drug List (NRDL), categorizing drugs into fully reimbursed (List A) and partially reimbursed (List B) categories, issued by the National Healthcare Security Administration.
Competitive Landscape
Key Players
Here are some of the major key players in the China Medication Access Programs Market:
- Gilead Sciences
- Takeda Pharmaceuticals
- Pfizer
- Novartis
- Merck
- AstraZeneca
- Bristol-Myers Squibb
- Sanofi
- Eli Lilly
- AbbVie
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Medication Access Programs Market Segmentation
By Disease Type
- Chronic
- Acute
By Therapeutic Areas
- Oncology
- Cardiology
- Rheumatology
- Others
By Patient Type
- Geriatric
- Pediatric
- Adult
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































