China LASIK Surgery Market Analysis
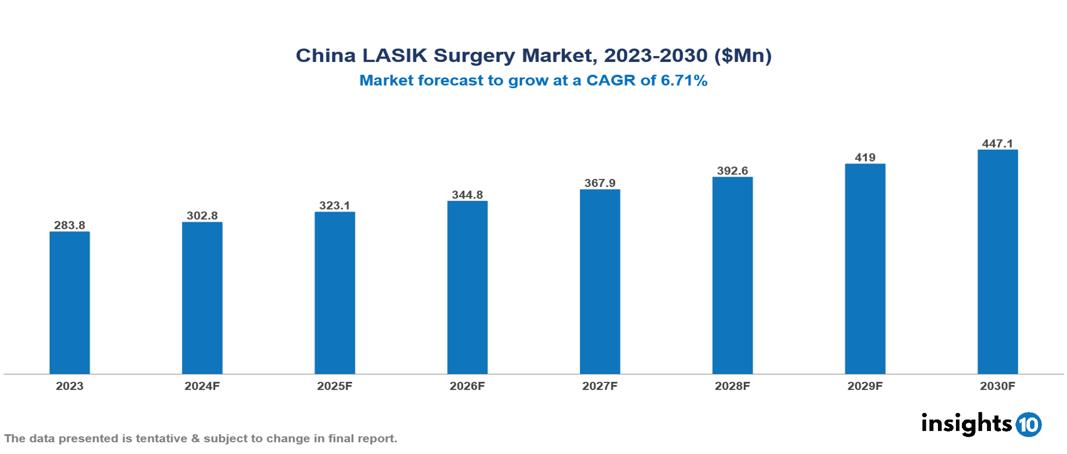
China LASIK Surgery Market was valued at $283.77 Mn in 2023 and is predicted to grow at a CAGR of 6.71% from 2023 to 2030, to $447.09 Mn by 2030. The key drivers of this industry include the rising prevalence of myopia, growing disposable income, and technological advancements. The industry is primarily dominated by Johnson & Johnson Vision, Zeimer Ophthalmic System AG, Abbott, and Carl ZEISS AG, among others.
Buy Now

China LASIK Surgery Market Executive Summary
China LASIK Surgery Market was valued at $283.77 Mn in 2023 and is predicted to grow at a CAGR of 6.71% from 2023 to 2030, to $447.09 Mn by 2030.
LASIK, or laser-assisted in situ keratomileusis, is a popular refractive surgery that reshapes the cornea to correct vision problems like near sightedness, farsightedness, and astigmatism, significantly reducing dependence on eyeglasses or contact lenses. Before surgery, an ophthalmologist performs a comprehensive eye exam to determine candidacy. The outpatient procedure, lasting about 15-30 minutes per eye, involves creating a thin corneal flap with a microkeratome or femtosecond laser, reshaping the underlying corneal tissue with an excimer laser, and repositioning the flap without stitches. Post-surgery, patients may experience temporary discomfort and blurred vision, with risks including dry eyes, glare, halos, infection, and rare vision under or overcorrections.
In 2019, the prevalence of moderate vision impairment in China was 2.57%, severe vision impairment was 0.25%, and blindness was 0.48%, with notable increases in moderate vision impairment by 133.67% and blindness by 64.35% from 1990 to 2019, primarily due to population aging. The pooled prevalence of dry eye disease in Asia was estimated at 20.1%, with higher rates in females (21.7%) compared to males (16.4%). Additionally, about 13.5% of China's population was aged 65 and above in 2021, leading to a rising trend in age-related eye diseases, as one in five individuals over 70 experienced some degree of vision loss. The market is therefore driven by significant factors like the rising prevalence of myopia, growing disposable income, and technological advancements. However, the high cost of surgery, limited access to qualified surgeons and facilities, and concerns about safety and quality restrict the growth and potential of the market.
A prominent player in this field is Johnson & Johnson Vision, Zeimer which announced a collaboration with Schwind eye-tech-solutions to develop a new femtosecond laser platform for various ophthalmic applications, including LASIK surgery on June 2023. Other contributors include Abbott, and Carl ZEISS AG among others.
Market Dynamics
Market Growth Drivers
Rising Prevalence of Myopia: China is experiencing a myopia epidemic, especially among young adults, with over 53% of those aged 16-24 affected. This significant population creates a high demand for corrective procedures like LASIK.
Growing Disposable Income: As China's economy continues to grow, many citizens are seeing an increase in disposable income. This financial flexibility encourages individuals to consider elective procedures like LASIK to enhance their quality of life, alongside a rising trend towards consumerism and personal health investment.
Technological Advancements: China is making significant strides in ophthalmic technology development. Innovations in laser technology and surgical methods lead to increased precision, quicker recovery times, and potentially lower complication rates, making LASIK a more appealing option for patients.
Market Restraints
High Cost of Surgery: LASIK surgery is quite costly in China, particularly about the average national income, presenting a significant barrier to access for many citizens.
Limited Access to Qualified Surgeons and Facilities: Availability of ophthalmologists trained in advanced LASIK techniques and well-equipped facilities is often concentrated in major cities, limiting access for individuals in rural areas.
Concerns about Safety and Quality: Historical issues regarding the safety and quality standards of some LASIK procedures in China have raised concerns. It is essential to build trust with qualified ophthalmologists and ensure compliance with stringent regulations to address these issues.
Regulatory Landscape and Reimbursement Scenario
China has a comprehensive regulatory framework overseeing LASIK surgery to ensure patient safety. The National Medical Products Administration (NMPA) regulates medical devices, including LASIK lasers, ensuring they meet safety and efficacy standards. The National Health Commission (NHC) sets health policies and guidelines related to ophthalmic procedures, covering pre-operative assessments and postoperative care. Additionally, the Chinese Medical Doctor Association (CMDA) provides ethical guidelines for medical practice, which LASIK surgeons must follow. Key regulations include strict pre-operative assessments, the use of approved medical devices, informed consent procedures, and licensing requirements for facilities and ophthalmologists.
Regarding reimbursement, LASIK surgery is typically considered elective in China, leading to limited public insurance coverage. Coverage may be available for severe refractive errors impacting daily life, but eligibility and extent vary by location and insurance plans. Some private health insurance plans may offer partial coverage, while most patients ultimately pay out of pocket for LASIK surgery. Costs can vary based on the surgeon's expertise, the technology used, and the facility, with financing options available at some LASIK clinics to help manage expenses.
Competitive Landscape
Key Players
Here are some of the major key players in the China LASIK Surgery
- Abbott
- Bausch Health Companies Inc.
- Ziemer Group AG
- Carl Zeiss Meditec AG
- Johnson & Johnson
- Nidek Co. Ltd.
- Topcon Corporation
- EyeCare Partners LLC
- Coherent Inc.
- LCA Vision Inc. dba
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China LASIK Surgery Market Segmentation
By Type
- Wavefront Optimized
- Wavefront-Guided
- Topography Guided
- All Laser
By Vision Error
- Myopia
- Hyperopia
- Astigmatism
- Others
By Product
- Excimer Laser
- Femtosecond Laser
By End-User
- Hospitals
- Eye Care Clinic
- LASIK Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































