China Cough Hypersensitivity Syndrome Therapeutics Market Analysis
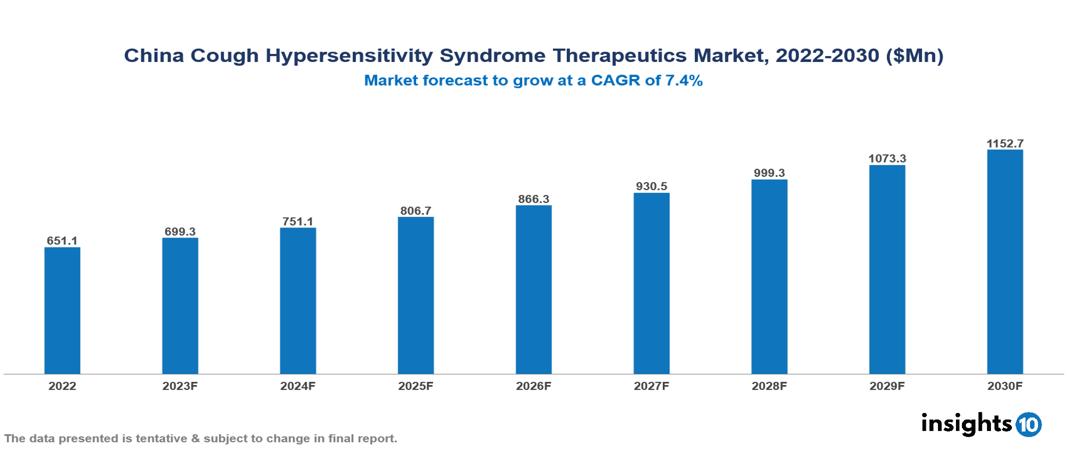
The China Cough Hypersensitivity Syndrome Therapeutics Market was valued at $0.651 Bn in 2022 and is predicted to grow at a CAGR of 7.4% from 2023 to 2030, to $1.153 Bn by 2030. The key drivers of this industry include the increasing prevalence of Cough Hypersensitivity Syndrome, the development of advanced therapeutics, and expanding healthcare infrastructure. The industry is primarily dominated by players such as Johnson & Johnson, GSK, Merck, Novartis, AstraZeneca, and Pfizer among others.
Buy Now

China Cough Hypersensitivity Syndrome Therapeutics Market Analysis Executive Summary
The China Cough Hypersensitivity Syndrome Therapeutics Market is at around $0.651 Bn in 2022 and is projected to reach $1.153 Bn in 2030, exhibiting a CAGR of 7.4% during the forecast period.
Cough Hypersensitivity Syndrome (CHS) is characterized by an exaggerated response to stimuli, leading to heightened sensitivity of the cough reflex. This increased sensitivity often results in persistent coughing triggered by various irritants such as dust, perfumes, or cold air. CHS can be caused by respiratory infections, gastroesophageal reflux disease (GERD), and exposure to environmental pollutants. Symptoms typically include a chronic cough, often without an apparent underlying respiratory condition, and may be accompanied by throat irritation or discomfort. Effective treatment for Cough Hypersensitivity Syndrome involves addressing the underlying causes and managing the exaggerated cough reflex. Treatment options include medications like antitussives, which suppress the cough reflex, and drugs targeting underlying conditions such as GERD. Non-pharmacological approaches, including behavioral therapies and speech therapy, may also be employed to modify the sensitivity of the cough reflex. Leading pharmaceutical companies like GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim are actively engaged in producing medications to treat CHS, offering a diverse range of options for effective management of this condition.
The estimated prevalence of chronic cough is around 7% of the population in China which is the major cause of China. The market is driven by key factors such as the increasing prevalence of Cough Hypersensitivity Syndrome and subsequently its risk factors like smoking and pollution, the development of advanced therapeutics, and expanding healthcare infrastructure. However, conditions such as lack of specific CHS treatment, limited awareness, and limited R&D impede the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Increasing prevalence of Cough Hypersensitivity Syndrome and contributing conditions: China is facing an escalating prevalence of chronic respiratory illnesses such as chronic obstructive pulmonary disease (COPD), asthma, and allergic rhinitis. The estimated overall prevalence of chronic cough is around 7% of the Chinese population. These ailments frequently give rise to CHS, prompting a surge in the need for diverse treatment solutions. Contributing to this upswing are factors like air pollution, smoking, and urbanization, which significantly affect vulnerable groups like children and the elderly.
Development of advanced therapeutics: Pharmaceutical companies are conducting research and development efforts to develop novel and effective treatments for CHS which offer improved treatment options, promoting market growth. These developments include inhaled corticosteroids with accurate delivery systems and antitussive drugs with increased efficacy.
Expanding healthcare infrastructure: China's healthcare infrastructure is expanding with increased access to specialists and treatment facilities in both urban and remote areas. This increased accessibility is boosting market expansion by allowing greater patient diagnosis and treatment. Government reimbursement policies for CHS therapies have a substantial impact on market growth by increasing affordability.
Market Restraints
Lack of specific treatment options: In China, there are presently no approved medications specifically designed for CHS. Available therapy options are used to address other disorders such as asthma or allergies, demonstrating limited efficacy and side effects. The lack of customized drugs results in treatment non-adherence, limiting market growth.
Limited awareness: Limited awareness of CHS, leads to underdiagnosis and delayed treatment. Inadequate understanding among patients and providers results in misdiagnosis, due to overlapping symptoms with other respiratory disorders like asthma or chronic cough. Poor access to healthcare facilities further contributes to slowing market growth.
Limited R&D: In contrast to other respiratory conditions, research focused on Cough Hypersensitivity Syndrome (CHS) is at an early stage. The insufficient investment in research and development may impede the identification and creation of novel, more efficient treatments specifically designed for CHS. Moreover, the limited collaboration among researchers, pharmaceutical firms, and healthcare providers can obstruct the translation of research discoveries into practical applications.
Healthcare Policies and Regulatory Landscape
The main regulatory body overseeing drugs and pharmaceuticals in China is the China National Medical Products Administration (NMPA). Formerly known as the China Food and Drug Administration (CFDA), NMPA is responsible for regulating the safety, efficacy, and quality of pharmaceuticals, medical devices, and cosmetics in the country. NMPA plays a crucial role in the approval process for new drugs, ensuring compliance with regulatory standards and protecting public health.
To obtain licensure for drugs and pharmaceuticals in China, companies must go through a rigorous approval process administered by NMPA. This process typically involves submitting comprehensive documentation on the product's safety, efficacy, and quality, along with the results of clinical trials conducted by Chinese regulations. NMPA conducts thorough reviews of the submissions to assess whether the product meets the required standards. Successful approval results in the issuance of a drug registration certificate, allowing the product to be marketed and sold in China.
The regulatory environment for new entrants is demanding, as stringent requirements are in place to ensure the safety and effectiveness of pharmaceutical products entering the Chinese market, creating a barrier to entry that requires significant investment in research, development, and regulatory compliance.
Competitive Landscape
Key Players
- Johnson & Johnson
- Bayer AG
- GlaxoSmithKline
- Pfizer
- Novartis
- AstraZeneca
- Teva Pharmaceuticals
- Boehringer Ingelheim
- Vertex Pharmaceuticals
- Merck
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Cough Hypersensitivity Syndrome Therapeutics Market Segmentation
By Drug Class
- Antitussive Agents
- Corticosteroids
- Short Acting Beta-2 Agonists
- Anti-cholinergic
- Antihistamines
- Proton Pump Inhibitors
- Others
By Route of Administration
- Oral
- Inhalation
- Others
By End Users
- Hospitals
- Speciality Clinics
- Homecare
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.