China Chronic Pain Therapeutics Market Analysis
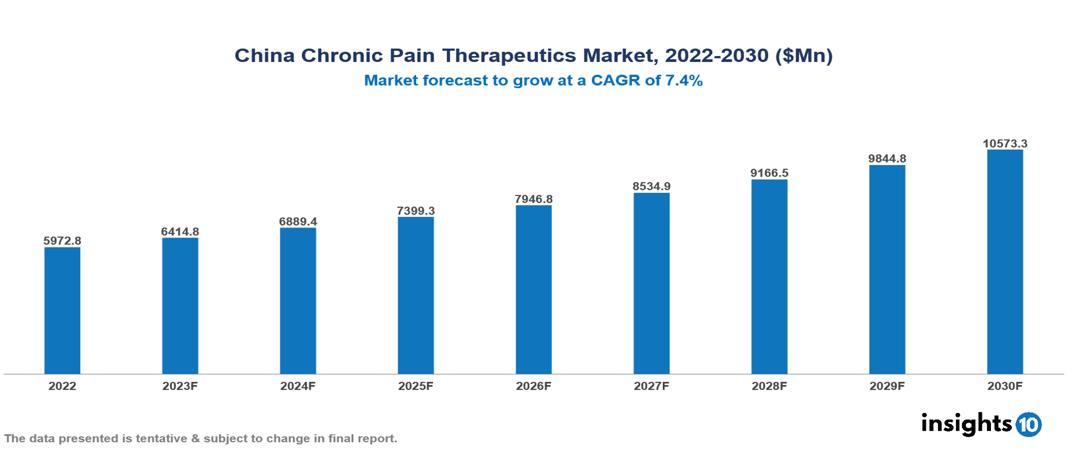
The China Chronic Pain Therapeutics Market is anticipated to experience a growth from $5.973 Bn in 2022 to $10.573 Bn by 2030, with a CAGR of 7.4% during the forecast period of 2022-2030. The key drivers of the chronic pain management market in China are the increased prevalence of chronic conditions, the aging population and associated comorbidities, increasing healthcare awareness, and the rising demand for non-opioid pain management alternatives. The China Chronic Pain Therapeutics Market encompasses various players across different segments, including Pfizer, Johnson & Johnson, Merck, Sanofi, AstraZeneca, Innovent Biologics, Shanghai Pharmaceuticals, Henlius Medical, BGI Genomics, Shanghai Fosun Pharma, etc., among various others.
Buy Now

China Chronic Pain Therapeutics Market Analysis Executive Summary
The China Chronic Pain Therapeutics Market is anticipated to experience a growth from $5.973 Bn in 2022 to $10.573 Bn by 2030, with a CAGR of 7.4% during the forecast period of 2022-2030.
Chronic pain refers to persistent and prolonged discomfort lasting beyond the anticipated healing period, typically exceeding three weeks. It can stem from various sources such as arthritis, fibromyalgia, back injuries, nerve damage, and inflammatory disorders, with factors like trauma, surgery, or diseases like cancer contributing to its development. Addressing chronic pain often involves a multidisciplinary approach, encompassing conventional methods like analgesics, physical therapy, and cognitive-behavioral therapy. Lifestyle adjustments, acupuncture, and alternative therapies may also be recommended. In the realm of pain management, technological advancements have introduced innovative strategies. Neurostimulation devices, such as spinal cord stimulators, utilize electrical impulses to modify pain signals. Intrathecal drug delivery systems enable precise medication administration directly into the spinal fluid. Virtual and augmented reality applications offer distraction therapy, alleviating the perception of pain. Additionally, regenerative medicine explores techniques like stem cell therapy for tissue repair. These emerging technologies, guided by an evolving understanding of chronic pain, contribute to a more personalized and effective landscape in the ongoing pursuit of enhanced pain treatment.
According to current estimates, more than 30% of the Chinese population suffers from chronic pain. The numbers show that males have a greater prevalence than females. In China, the most prevalent causes of chronic pain include musculoskeletal pain, neuropathic pain, headache, cancer-related pain, and fibromyalgia. Factors that may contribute to chronic pain in China include demographic features, health status, and health practices.
The key drivers of the chronic pain management market in China are the increased prevalence of chronic conditions, the aging population and associated comorbidities, increasing healthcare awareness, and the rising demand for non-opioid pain management alternatives.
Pfizer and Johnson & Johnson have the highest brand awareness in China due to their lengthy history and substantial marketing activities. They provide a wider selection of items on the market. Local companies such as Henlius Medical, BGI Genomics, and Shanghai Fosun Pharma (via collaborations) have a high potential for future growth owing to their emphasis on innovative techniques and strategic alliances.
Market Dynamics
Market Growth Drivers
Increased prevalence: The prevalence of chronic conditions in China, including arthritis, musculoskeletal disorders, and cancer, is on the rise, contributing significantly to the burden of chronic pain. The aging population, coupled with an increase in comorbidities, is driving demand for effective solutions in pain management.
Increasing awareness: China is experiencing a notable surge in healthcare awareness, propelled by government initiatives and media exposure, particularly in the realm of chronic pain management. The growing healthcare spending power among the population further encourages patients to explore and adopt innovative therapies for pain relief.
Increasing demand for alternatives: Amid concerns about opioid addiction and associated side effects, both patients and healthcare providers in China are increasingly inclined towards non-opioid pain management options. This shift opens up opportunities for diverse therapeutic approaches, including the utilization of neuromodulation devices, minimally invasive procedures, and targeted drug delivery systems, reflecting a broader trend in the country's approach to addressing chronic pain.
Market Restraints
Lack of reimbursement: The healthcare insurance system in China predominantly provides reimbursement for essential drugs and standard procedures, often excluding newer and innovative therapies for chronic pain. This results in affordability challenges for patients and poses barriers to market entry for emerging solutions.
Regulatory hurdles: Navigating China's intricate regulatory framework for drug and device approval is a time-consuming and costly process, creating obstacles for both foreign and domestic companies seeking to introduce advanced pain management options. This stringent regulatory environment can significantly delay the availability of innovative therapies in the market.
Lack of specialists: China grapples with a shortage of pain specialists and comprehensive pain management centers, particularly in rural areas. This scarcity limits access to specialized care, impeding the optimal utilization of existing therapeutic options for individuals experiencing chronic pain.
Healthcare Policies and Regulatory Landscape
China's healthcare policies and drug regulatory agencies play pivotal roles in shaping the nation's healthcare landscape. The healthcare policies in China are guided by a commitment to ensuring universal access to basic healthcare services for its vast population. The government has implemented various initiatives to improve healthcare infrastructure, enhance the quality of medical services, and expand insurance coverage. The National Medical Products Administration (NMPA), serves as the key regulatory authority overseeing drug and medical device approvals. NMPA plays a crucial role in safeguarding public health by regulating the safety, efficacy, and quality of pharmaceuticals and healthcare products. The agency enforces rigorous standards for the approval and marketing of drugs, ensuring compliance with international best practices. China's drug regulatory landscape is characterized by a complex and evolving framework. The NMPA is responsible for evaluating and approving new drugs, conducting inspections, and overseeing post-marketing surveillance. The agency's commitment to enhancing regulatory transparency and streamlining approval processes reflects China's aspiration to become a global player in the pharmaceutical industry.
Competitive Landscape
Key Players:
- Pfizer
- Johnson & Johnson
- Merck
- Sanofi
- AstraZeneca
- Innovent Biologics
- Shanghai Pharmaceuticals
- Henlius Medical
- BGI Genomics
- Shanghai Fosun Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Chronic Pain Therapeutics Market Segmentation
By Indication
- Neuropathic Pain
- Back Pain
- Headaches
- Arthritis Pain
- Muscular Pain
- Idiopathic Pain
- Others
By Drug Class
- Analgesics
- Opioids
- NSAIDs
- Anaesthetics
- Others
By Route of Administration
- Oral
- Topical
- Parenteral
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.