China Cell Based Immunotherapy Market Analysis
China Cell Based Immunotherapy Market is projected to grow from $xx Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022 - 2030. The increasing incidence of cancer, advancements in cell therapy technologies, a favourable regulatory environment, growing investments and collaborations, the trend toward personalised medicine, positive clinical outcomes, and patient demand for effective and targeted treatments are driving the cell-based immunotherapy market. Novartis, Gilead Sciences, Bristol Myers Squibb, bluebird bio, Adaptimmune Therapeutics, Cellectis, and Precision Biosciences are among the leading global players.
Buy Now

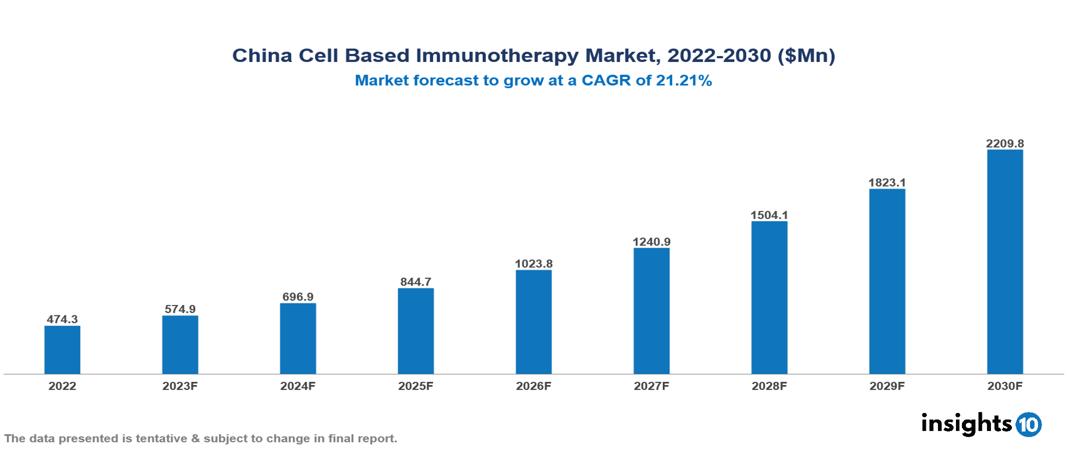
China Cell Based Immunotherapy Market Analysis Summary
China Cell Based Immunotherapy Market is valued at around $474.3 Mn in 2022 and is projected to reach $2209.8 Mn by 2030, exhibiting a CAGR of 21.21% during the forecast period 2023-2030.
Cell-based immunotherapy employs an immune system cell type as a medicinal agent. The increasing incidence of cancer, advancements in cell therapy technologies, a favorable regulatory environment, growing investments, and collaborations, the trend toward personalized medicine, positive clinical outcomes, and patient demand for effective and targeted treatments drive the cell-based immunotherapy market. These forces contribute to discovering and marketing novel cell-based immunotherapies, providing new therapeutic options for various diseases, including cancer, and powering the field's rapid expansion and advancement. Cell-based immunotherapy, also known as cellular immunotherapy or cell therapy, is the use of living cells to treat diseases such as cancer. Prominent global players include Novartis, Gilead Sciences, Bristol Myers Squibb, Bluebird Bio, Adaptimmune Therapeutics, Cellectis, and Precision Biosciences are among the leading global players.
Market Dynamics
Market Growth Drivers
- Rising Cancer Incidence: As the global frequency of cancer rises, so does the demand for effective and focused therapy. Cell-based immunotherapies, such as chimeric antigen receptor (CAR) T-cell treatment and T-cell receptor (TCR) therapy, have shown encouraging outcomes in treating leukemia, lymphoma, and solid tumors
- Technological Advances in Cell Therapy: Cell therapy technology breakthroughs, including as genetic engineering techniques and stem cell research, have greatly increased the potential applications of cell-based immunotherapy. These breakthroughs have enabled the development of more accurate and tailored treatments
- Regulatory Environment Favorable: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have recognized the potential of cell-based immunotherapies and have expedited regulatory routes
- Increasing Investments and Collaborations: Pharmaceutical corporations, biotechnology firms, and research institutions have made significant investments in the cell-based immunotherapy market. Collaborations between academic institutions, industrial companies, and government organizations have also aided in this field's research and development, resulting in the introduction of new medicines and treatment techniques
- Personalized Medicine Is Becoming More Popular: Cell-based immunotherapies use a patient's cells or genetically modified cells to provide a tailored approach to treatment. Cell-based immunotherapies are predicted to acquire market traction as the emphasis shifts to customized medicine and precision medicines
Market Restraints
- The high cost of therapy is owing to the intricacy of the manufacturing process, which includes cell separation, expansion, and genetic alteration. The high costs connected with these medicines make widespread adoption and reimbursement difficult
- Limited Availability: Currently, authorized cell-based immunotherapies are only available in a few indications. Increasing the number of diseases that can be treated and producing off-the-shelf cell treatments would improve accessibility and uptake
- Safety Concerns: As cell-based immunotherapies involve the manipulation of living cells, there are potential safety concerns, including cytokine release syndrome (CRS) and neurotoxicity. Ongoing research and development efforts are focused on optimizing safety profiles and minimizing adverse events associated with these therapies
- Infrastructure and Logistics: The implementation of cell-based immunotherapies requires specialized infrastructure and expertise, including manufacturing facilities and skilled personnel. Building and scaling up these capabilities can be challenging, particularly in regions with limited resources
Competitive Landscape
Key Players
- Novartis
- Gilead Sciences
- Bristol Myers Squibb
- Bluebird bio
- Adaptimmune Therapeutics
- Cellectis
- Precision Biosciences
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
sexy Cell-Based Immunotherapy Market Segmentations
By Therapy Type
- CAR-T Cell Therapy
- TCR Therapy
- TIL Therapy (Tumor-Infiltrating Lymphocytes)
- NK Cell Therapy (Natural Killer Cells)
- Dendritic Cell Therapy
- Others
By Application
- Cancer Treatment
- Autoimmune Diseases
- Infectious Diseases
- Genetic Disorders
- Others
By End-User
- Hospitals and Clinics
- Research Institutes and Academic Centers
- Biopharmaceutical Companies
- Contract Development and Manufacturing Organizations (CDMOs)
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































