China Brugada Syndrome Market Analysis
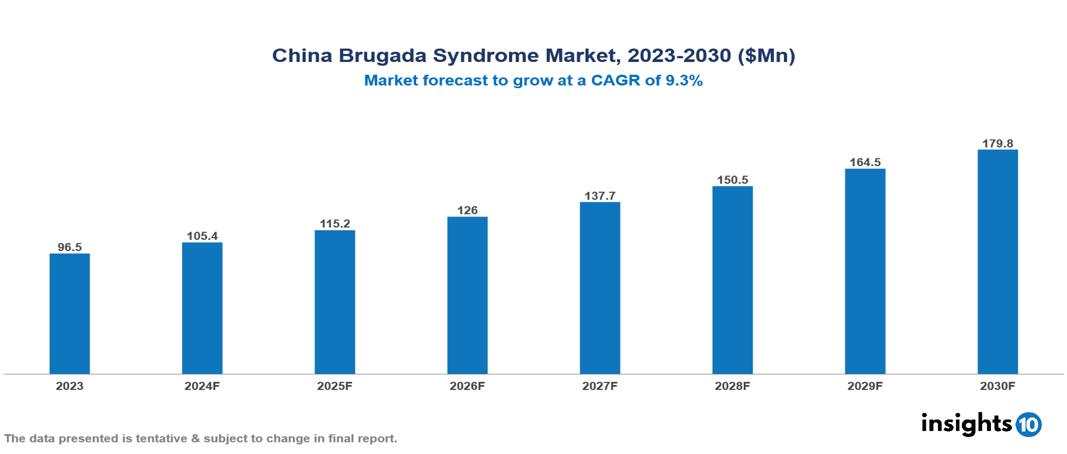
The China Brugada Syndrome Market was valued at $96.5 Mn in 2023 and is predicted to grow at a CAGR of 9.3% from 2023 to 2030, to $179.8 Mn by 2030. The key drivers of the market include growing prevalence of cardiovascular diseases, increasing awareness and diagnosis, and advancements in genetic testing. The prominent players of the China Brugada Syndrome Market are AstraZeneca, Abbott, Bayer, Merck, and Sanofi, among others.
Buy Now

China Brugada Syndrome Market Executive Summary
The China Brugada Syndrome Market is at around $96.5 Mn in 2023 and is projected to reach $179.8 Mn in 2030, exhibiting a CAGR of 9.3% during the forecast period.
Brugada syndrome is a rare, but potentially threatening, genetic condition that causes abnormal electrical activity in the heart, leading to an increased risk of sudden cardiac death. People with Brugada syndrome have an increased risk of irregular heart rhythms beginning in the lower chambers of the heart, i.e., the ventricles. Common signs and symptoms associated with Brugada Syndrome include dizziness, fainting, gasping and laboured breathing, particularly at night, irregular heartbeats or palpitations, extremely fast and chaotic heartbeat, and seizures. The risk factors for Brugada syndrome include family history of Brugada syndrome, being male, race, and fever.
The China Brugada Syndrome Market is driven by significant factors such as growing prevalence of cardiovascular diseases, increasing awareness and diagnosis, and advancements in genetic testing. However, high cost of treatment, side effects and complications of treatment, and limited R&D restrict the growth and potential of the market.
The major players of the China Brugada Syndrome Market are AstraZeneca, Abbott, Bayer, Merck, and Sanofi, among others.
Market Dynamics
Market Growth Drivers
Growing Prevalence of Cardiovascular Diseases: It is estimated that approximately 330 Mn individuals in China are affected by cardiovascular diseases. With cardiovascular conditions becoming more prevalent due to aging populations, lifestyle changes, and increasing rates of hypertension and diabetes, there is a rising awareness and focus on genetic disorders like Brugada syndrome. This growing prevalence drives more investments in diagnostic tools, research, and advanced treatments for Brugada syndrome, as healthcare systems aim to tackle the wider range of cardiovascular health issues. As a result, the demand for effective management and therapeutic options for Brugada syndrome is expected to increase, thereby driving market growth.
Increasing Awareness and Diagnosis: Raising awareness and improving the diagnosis of Brugada syndrome are vital for effectively managing this potentially fatal condition. Greater awareness among healthcare professionals and the public leads to more frequent and earlier diagnoses. Educational campaigns, training programs for medical staff, and public health initiatives are essential in spreading knowledge about the symptoms, risks, and genetic aspects of Brugada syndrome. Overall, increased awareness and better diagnostic capabilities lead to the market growth.
Advancements in Genetic Testing: Advancements in genetic testing significantly drive the growth of the Brugada syndrome market. The development of more advanced and affordable genetic testing technologies has made it easier to identify individuals with Brugada syndrome or those at risk. Enhanced accuracy and accessibility of these tests enable earlier diagnosis, allowing for timely intervention and better management of the condition. As genetic testing becomes more integrated into routine healthcare, awareness among healthcare providers and patients increases, leading to a higher rate of diagnosis. This increase in early and accurate identification of Brugada syndrome patients boosts the demand for specialized treatments and monitoring solutions, thereby propelling market growth.
Market Restraints
High Cost of Treatment: The high cost of treatment and diagnostic tools for Brugada syndrome poses a substantial obstacle to market growth. Advanced interventions, such as implantable cardioverter defibrillators (ICDs) and specialized genetic testing, are often prohibitively expensive, making them inaccessible to many patients, especially those in low- and middle-income groups. This financial barrier limits access to crucial care and treatment, hindering widespread diagnosis, early intervention, and effective management of Brugada syndrome, thereby slowing the market's expansion.
Side Effects and Complications of Treatments: The market growth for Brugada syndrome treatments is significantly hindered by the side effects and complications associated with therapies like implantable cardioverter defibrillators (ICDs). Patients may experience infections, device malfunctions, psychological impacts, and other adverse effects, which can deter them from choosing these treatments. These potential risks and complications lead to reduced acceptance and adoption of advanced therapies, thereby restricting market expansion despite the availability of effective treatment options.
Limited R&D: The limited research and development in Brugada syndrome significantly hinders market growth. As a relatively rare condition, it attracts less investment compared to more common cardiovascular diseases, resulting in slower progress in understanding the disease, developing new diagnostic tools, and creating innovative treatments. Consequently, medical breakthroughs are less frequent, reducing the availability of effective therapies and diagnostic methods, and ultimately restricting the market growth for Brugada syndrome.
Regulatory Landscape and Reimbursement Scenario
China’s pharmaceutical regulatory authority was previously called China Food and Drug Administration (CFDA) which was renamed to National Medicinal Products Administration (NMPA) in 2018. The NMPA is the government agency which is responsible for regulating the pharmaceuticals, cosmetics, medical devices, and in-vitro diagnostics in the nation.
The effectiveness and safety of medications, medical equipment, and cosmetics sold in China are ensured by the NMPA. This entails checking applications, carrying out examinations, and monitoring post-market risks. Also, the whole medical product life cycle, from research and development to manufacture, marketing, and distribution, is governed by rules that are created and enforced by the NMPA. The Chinese Pharmacopoeia, which establishes quality standards for pharmaceuticals, is one of the requirements they set for the safety and quality of medicinal products. By expediting the approval procedures for cutting-edge medications and equipment, the NMPA promotes research and development of new and improved medical products.
The NRDL is the main pathway for pharmaceutical reimbursement in China, with primary goal being to improve affordability of drug treatments. The reimbursement landscape for pharmaceuticals in China is complex and prioritizes cost-effectiveness. Even though there are several advantages to being an NRDL member, there are competition in the process and significant cost savings. It is essential for pharmaceutical companies doing business in China to comprehend this framework.
Competitive Landscape
Key Players
Here are some of the major key players in the China Brugada Syndrome Market:
- AstraZeneca
- Abbott
- Bayer
- Merck
- Sanofi
- Medtronic
- GE Healthcare
- Boston Scientific Corporation
- Pfizer
- Glaxosmithkline
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Brugada Syndrome Market Segmentation
By Diagnosis
- Electrocardiogram
- Electrophysiology (Ep) Test
- Genetic Testing
By Treatment
- Implantable Cardioverter-Defibrillator
- Drug Therapy
By End User
- Hospitals
- Clinics
- Diagnostic Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































