China Bio-implant Market Analysis
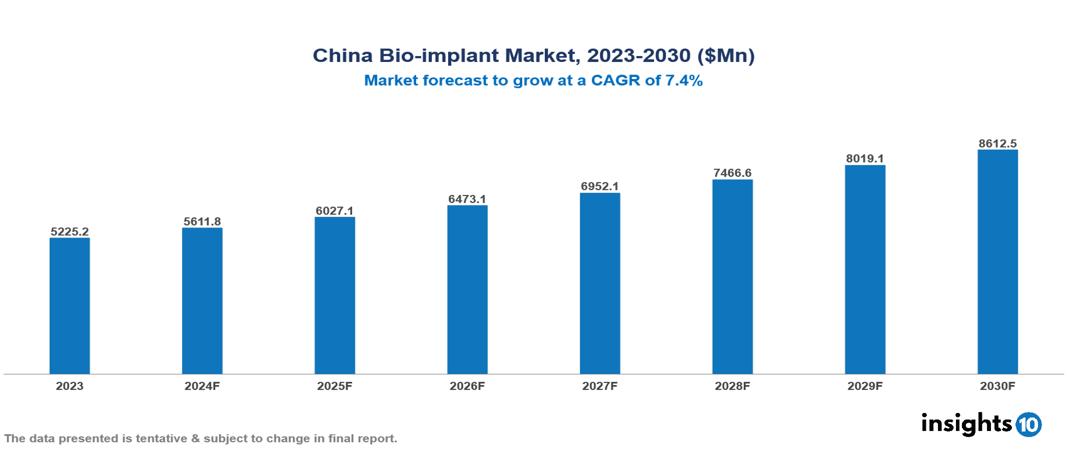
The China Bio-implant Market was valued at $5225.2 Mn in 2023 and is predicted to grow at a CAGR of 7.4% from 2023 to 2030, to $8612.5 Mn by 2030. The China Bio-implant Market is growing due to Growing Medical Tourism, Rising Chronic Disease Prevalence, Urbanization, and Lifestyle Changes. The market is primarily dominated by players such as MicroPort, Zimmer Biomet, Stryker Boston Scientific Corporation, Otto Bock Holding GmbH & Co. KG, Medtronic plc, Boston Scientific Corporation, and Johnson & Johnson Services.
Buy Now

China Bio-implant Market Executive Summary
China Bio-implant Market is at around $5225.2 Mn in 2023 and is projected to reach $8612.5 Mn in 2030, exhibiting a CAGR of 7.4% during the forecast period.
A biological structure is intended to be replaced, improved, or supplemented by prosthetic devices known as bio-implants. They are created using a variety of biosynthetic materials, including collagen and tissue-engineered products like artificial skin. Because bio-implants are often composed of biocompatible materials, rejection is less common. The bio-implant sector has grown significantly over time as a result of advancements in healthcare technology. The market for bio-implants has become increasingly complex due to the emergence of critical medical diseases, despite the fact that the healthcare industry has witnessed substantial technical developments over time.
In China, chronic diseases such as cardiovascular conditions and diabetes affect over 300 Mn people, driven by urbanization and lifestyle changes. The aging population, with over 250 Mn aged 60 and above, underscores increasing demand for bioimplants like orthopedic and dental implants. Demographically, urbanization at 60% promotes healthcare accessibility but also heightens chronic disease rates. Therefore, the market is driven by significant factors like Growing Medical Tourism, Rising Chronic Disease Prevalence, Urbanization, and Lifestyle Changes. However, Competitive landscape, Regulatory challenges, and Cost of treatment restrict the growth and potential of the market.
MicroPort has been actively developing and commercializing innovative products such as hip and knee implants. They have also focused on enhancing their research and development capabilities to introduce advanced technologies that cater to the growing demand for joint replacement surgeries in China.
Market Dynamics
Market Growth Drivers
Growing Medical Tourism: China is increasingly becoming a destination for medical tourism, attracting patients seeking high-quality and cost-effective medical treatments, including bioimplant procedures. Medical tourism revenue in China was estimated to be USD 426 billion in 2022.
Rising Chronic Disease Prevalence: Chronic diseases like osteoarthritis and cardiovascular conditions are prevalent among China's aging population, necessitating bioimplant interventions. The prevalence of osteoarthritis among Chinese adults aged 60 and above was estimated at 21.6% in a study published by the National Center for Biotechnology Information.
Urbanization and Lifestyle Changes: Urbanization at 60% and lifestyle changes in China are influencing healthcare patterns, including the demand for bioimplants. Urban residents, exposed to higher levels of pollution and sedentary lifestyles, are more prone to chronic diseases and injuries necessitating implant treatments.
Market Restraints
Competitive landscape: China is home to a growing number of domestic and international companies competing for market share. This competition drives innovation but also intensifies pricing pressures and challenges profitability. Local manufacturers often face competition from established global players, necessitating continuous investment in research and development to stay competitive.
Regulatory challenges: The regulatory framework for medical devices, including bioimplants, is stringent and constantly evolving. Manufacturers must navigate complex approval processes and adhere to stringent quality standards, which can delay product launches and increase costs. Moreover, regulatory changes aimed at enhancing safety and efficacy can sometimes pose additional hurdles to market entry and expansion.
Cost of treatment: Bioimplants, including dental implants and orthopedic implants, are often expensive due to the advanced technology and materials involved. For instance, the average cost of a dental implant procedure in China can range from $1,550 to $4,650, which is prohibitive for many potential patients, especially in a country where significant portions of the population do not have adequate health insurance coverage for such procedures. This high cost acts as a barrier to entry for many patients seeking these treatments, thereby limiting market growth.
Regulatory Landscape and Reimbursement scenario
The National Medical Products Administration (NMPA) regulates the entire lifecycle of bioimplants from research and development to manufacturing, marketing, and post-market surveillance. Foreign manufacturers must also comply with China's regulations, often requiring local partnerships or subsidiaries for market entry. The regulatory framework aims to ensure product safety, efficacy, and quality, reflecting China's commitment to improving healthcare standards and patient outcomes. Changes in regulations, such as updates to clinical trial requirements or post-market surveillance protocols, impact market entry strategies and ongoing compliance for companies operating in China's bioimplant sector.
Despite a growing demand driven by an aging population and increasing chronic disease prevalence, reimbursement challenges persist. High out-of-pocket costs and limited coverage under public health schemes hinder widespread affordability and utilization. These factors create barriers for patients and healthcare providers alike, limiting market expansion and innovation. Addressing these reimbursement gaps through policy adjustments and expanding coverage could significantly enhance market growth and patient access to essential bioimplant technologies in China.
Competitive Landscape
Key Players
Here are some of the major key players in the China Bio-implant Market:
- MicroPort
- Zimmer Biomet
- Stryker
- Neodent
- Ossur
- Boston Scientific Corporation
- Otto Bock Holding GmbH & Co. KG
- Medtronic
- Boston Scientific Corporation
- Johnson & Johnson Services, Inc.
- LifeNet Health
- Smith & Nephew
- Arthrex, Inc.
- Clinic Lemanic
- Alpha Bio Tec
- MiMedx Group
- St Jude Medical (Abbott)
- DePuy Synthes
- Exactech, Inc.
- Cochlear Ltd
- Straumann AG
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Bio-implant Market Segmentation
By Material
- Ceramics
- Polymers
- Alloys
- Biomaterials Metals
By Type
- Dental Bio-implants
- Orthopedic Bio-implants
- Spinal Bio-implants
- Ophthalmology Bio-implants
- Cardiovascular Bio-implants
- Others
By Mode of Administration
- Surgical
- Injectable
By End User
- Hospitals
- Speciality Clinics
- Ambulatory surgical centers
By Origin
- Autograft
- Allograft
- Xenograft
- Synthetic
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































