China Adult Malignant Glioma Therapeutics Market Analysis
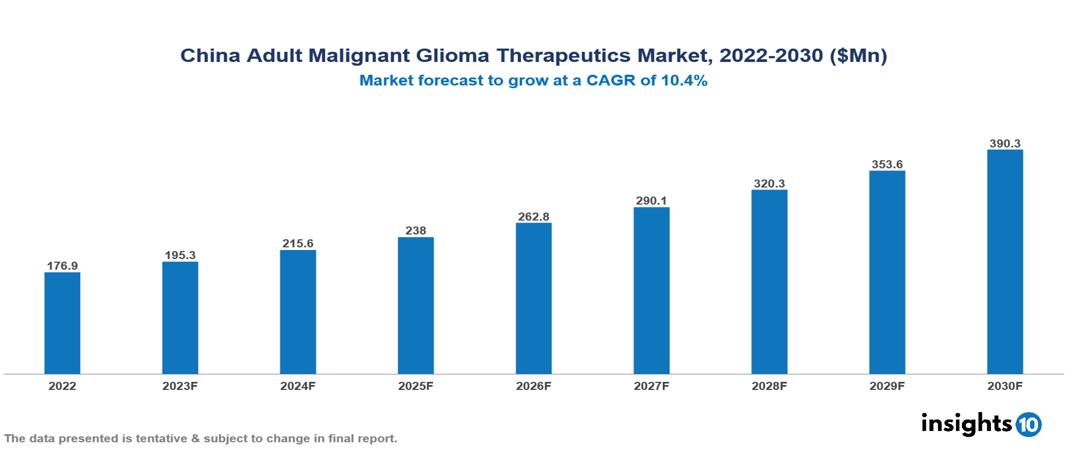
The China Adult Glioma Therapeutics Market is valued at around $177 Mn in 2022 and is projected to reach $390 Mn by 2030, exhibiting a CAGR of 10.4% during the forecast period. The rising prevalence of gliomas, driven by an aging population, improved diagnostic capabilities, and urbanization, further amplifies the demand for diverse and effective treatment options. Key players in the China Adult Malignant Glioma Therapeutics Market include companies like Merck & Co., Roche, Bristol-Myers Squibb, Novartis, Pfizer, Innovent Pharmaceuticals, BeiGene, Shanghai Junshi Biosciences, Hutchison China MediTech (Chi-Med) etc
Buy Now

China Adult Malignant Glioma Therapeutics Market Executive Summary
The China Adult Glioma Therapeutics Market is valued at around $177 Mn in 2022 and is projected to reach $390 Mn by 2030, exhibiting a CAGR of 10.4% during the forecast period.
Adult Malignant Gliomas are a particular kind of tumour that affects the brain and spinal cord. Gliomas can occur in the brain and in various locations in the nervous system, including the brain stem and spinal column. The kind of glial cells from which gliomas develop and their level of aggressiveness determine how these tumours are categorized. Astrocytes, oligodendrocytes, and ependymal cells are the three primary subtypes of glial cells. The most prevalent kind of glioma is an astrocyte-derived tumour called an astrocytoma. Glioblastoma, which is often referred to as glioblastoma multiforme, is a highly aggressive brain tumour that develops from the brain's glial cells. Among gliomas, it is the most aggressive and lethal kind, making up around 15% of all primary brain tumours and 50% of all gliomas. The size, location, WHO grade, and subtype of a glioma all have a significant impact on the course of treatment. Treatment options include surgery, radiation therapy, chemotherapy, and targeted molecular therapy.
China has an age-standardized prevalence of primary brain tumour of 25.43 per 100,000 people, creating a very large pool of glioma patients. Roughly 34.1% of these are categorized as gliomas. For both men and women, the incidence of lymphoma rises in their fifth and sixth decades of life. However, with a male-to-female ratio ranging from 1.5:1 to 2.2:1, men are somewhat more likely than women to acquire gliomas. The rising prevalence of gliomas, driven by an aging population, improved diagnostic capabilities, and urbanization, further amplifies the demand for diverse and effective treatment options.
With temozolomide (Temodal), the typical first-line therapy for glioblastoma multiforme (GBM), the most aggressive type of glioma, Merck & Co. continues to hold a leading position in the China Adult Malignant Glioma Therapeutic Market. A local pharmaceutical company, Innovent Pharmaceuticals, stands out as a serious competitor because of its late-stage pipeline development of PD-1 inhibitors (Sintilimab) and EGFR TKI (Icotinib).
Market Dynamics
Market Growth Drivers
Changing Treatment Landscape: Targeted medicines are becoming more and more important. Approaches to personalized medicine show potential for better results and growing markets. Immunotherapy is becoming more popular. Treatment options for glioblastoma appear to include PD-1 inhibitors such as tislelizumab (BeiGene) and sintilimab (Innovent). The standard of care is increasingly combining surgery, radiation, and targeted/immunotherapy medications, which is generating demand for a variety of treatment choices on the market.
Government Support: Proposals such as the "Healthy China 2030" plan provide priority to cancer control and prevention, which may increase glioma treatments' accessibility and research and development. The expedited medication clearance procedures implemented by China's Medication Regulatory Bureau have benefited local businesses and opened up markets for novel drugs.
Increased Disease Prevalence: One major contributing factor is the aging population, as the incidence of gliomas peaks in later life. The elderly population in China is driving market expansion. Early diagnosis and a greater case count are the results of improved and expanded access to sophisticated imaging technology like MRI. China's urbanization is promoting early detection. Glioma incidence is higher in urban than rural locations, possibly as a result of lifestyle and environmental variables.
Market Restraints
Affordability: Temozolomide and Avastin, two cutting-edge glioma medications, come with hefty price tags that prevent many patients from using them. Even with better healthcare coverage, out-of-pocket costs for cancer treatment are still high in China. The high expense of medical care is a challenge for those without insurance.
Uneven Distribution: Disparities in access to cutting-edge therapies are brought about by the unequal distribution of healthcare resources, especially in rural regions. Opportunities are created by urbanization, but because rural regions are still heavily populated and lack of access to technology, their demands are not being satisfied. Access is further limited in rural hospitals by a shortage of highly skilled professionals and cutting-edge medical equipment.
Lack of Awareness: Delays in diagnosis can occur when glioma symptoms and risk factors are not understood, frequently resulting in an advanced stage of the illness. The prognosis is poorer overall, and treatment efficacy is greatly reduced by this delay. People are less likely to seek medical attention or follow treatment plans if they are ignorant about treatment alternatives or their advantages. This results in decreased demand for glioma treatments, which might impede market growth and make these medications less accessible to individuals who most need them.
Healthcare Policies and Regulatory Landscape
Over 95% of the population in China is covered by the extensive public healthcare system (Urban Employee Basic Medical Insurance and New Rural Cooperative Medical Scheme). This includes the foundational knowledge for several treatments, including many therapies. The government places a high priority on primary care services and facilities, accessibility, and early sickness identification for conditions. Drug approval, clinical research, and market surveillance are within the purview of the National Medical Products Administration (NMPA), previously the China Food and Drug Administration (CFDA). Even though its procedures and regulations are getting better, foreign businesses may still find them drawn out and challenging. All manufacturers of pharmaceuticals, whether local or foreign, must adhere to strict GMP standards. Lengthy approval procedures and challenging application processes may delay the release of new medications. Comprehending China's intricate healthcare laws and regulations necessitates meticulous planning, astute teamwork, and a commitment to creating affordable, inventive solutions that specifically address the needs of the Chinese market.
Competitive Landscape
Key Players
- Merck & Co.
- Roche
- Bristol-Myers Squibb
- Novartis
- Pfizer
- Innovent Pharmaceuticals
- BeiGene
- Shanghai Junshi Biosciences
- Hutchison China MediTech (Chi-Med)
- Akeso
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
China Adult Malignant Glioma Therapeutics Market Segmentation
By Disease Type
- Glioblastoma Multiforme
- Anaplastic Astrocytoma
- Anaplastic Oligodendroglioma
- Anaplastic Oligoastrocytoma
- Other Types
By Treatment Type
- Chemotherapy
- Targeted Drug Therapy
- Radiation Therapy
- Surgery
- Gene Therapy
- Immunotherapy
- Vaccines
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Pharmacies
By Disease Stage
- Early-Stage Tumour
- Late-Stage Tumour
- Palliative Care
By Route of Administration
- Oral
- Parenteral
- Others
By End User
- Hospitals
- Speciality Clinics
- Chemotherapy Centres
- Radiotherapy Centres
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.