Canada Glioma Therapeutics Market Analysis
Canada Glioma Therapeutics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. Brain and spinal cord tumours called gliomas attack the glial cells in the brain. Positive regulatory environments and the rising prevalence of gliomas are two variables that are expected to drive market expansion. Having a robust pipeline is anticipated to be a key factor in driving the market for glioma therapeutics during the course of the projected period. Over the course of the projected period, it is anticipated that the prevalence of brain tumours would spur market expansion. Global companies in the Glioma Therapeutics Market are Thermo Fisher Scientific Inc., Emcure Pharmaceuticals Ltd., Sigma-Aldrich Co., Pfizer Inc, Taj Pharmaceuticals Limited, Teva Pharmaceutical Industries Ltd., GE Healthcare, Siemens Healthineers, Sun Pharmaceutical Industries, Philips Healthcare, Shimadzu Corporation, Toshiba Medical Systems Corporation, Merck & Co., Inc., F. Hoffmann-Le Roche AG, Arbor Pharmaceuticals LLC Ltd., Novartis International AG, Amneal Pharmaceuticals. LLC, AstraZeneca, Carestream Health, Hitachi Medical Corporation.
Buy Now

Canada Glioma Therapeutics Market Analysis Summary
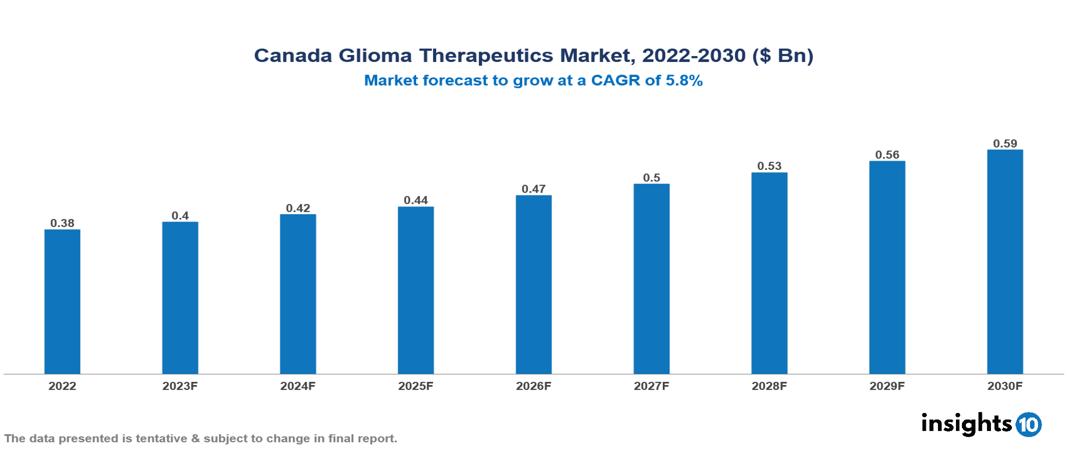
Canada Glioma Therapeutics Market is valued at around $0.38 Bn in 2022 and is projected to reach $0.59 Bn by 2030, exhibiting a CAGR of 5.8% during the forecast period 2023-2030.
The brain and spinal cord can develop gliomas, a specific type of tumour. Gliomas form in the gluey support cells (glial cells), which encircle and support nerve cells.
Glioma frequently exhibits the following signs and symptoms: headache, nausea or vomiting, dizziness or deterioration in mental function, memory loss, urine incontinence, vision issues, speech difficulties, seizures, etc. The market is expected to develop as a result of a number of factors, including the rising prevalence of glioblastoma multiforme, increased R&D, and favourable regulatory environments. During the projected period, a robust pipeline is anticipated to play a significant role in driving the glioblastoma multiforme (GBM) therapy market. The increasing incidence of brain tumours is expected to boost the growth of the market over the forecast period.
Global companies in the Glioma Therapeutics Market are Thermo Fisher Scientific Inc., Emcure Pharmaceuticals Ltd., Sigma-Aldrich Co., Pfizer Inc, Taj Pharmaceuticals Limited, Novartis International AG, Teva Pharmaceutical Industries Ltd., GE Healthcare, Siemens Healthineers, Philips Healthcare, Shimadzu Corporation, Toshiba Medical Systems Corporation, Merck & Co., Inc., F. Hoffmann-Le Roche AG, Arbor Pharmaceuticals, LLC, Sun Pharmaceutical Industries, Ltd., Amneal Pharmaceuticals. LLC, AstraZeneca, Carestream Health, Hitachi Medical Corporation, and others.
Market Dynamics
Market Drivers
One of the main factors driving the rise in the elderly population is the fact that glioma prevalence rates rise with age, especially between the ages of 45 and 65. The World Health Organization (WHO) predicts a 120 per cent growth in the number of people over 65 from 702.9 million in 2019 to 1,548.9 million in 2050. This increasing rate is alarming because the mortality rate from this illness has sharply increased. Treatments and drugs that increase patient longevity and decrease the incidence of patient fatalities are required as a result of this growth. Rising demand for these drugs is the main reason propelling the worldwide glioma market's revenue growth. One of the main factors driving the rise in the elderly population is the fact that glioma prevalence rates rise with age, especially between the ages of 45 and 65. The World Health Organization (WHO) predicts a 120 per cent growth in the number of people over 65 from 702.9 million in 2019 to 1,548.9 million in 2050. This increasing rate is alarming because the mortality rate from this illness has sharply increased. Treatments and drugs that increase patient longevity and decrease the incidence of patient fatalities are required as a result of this growth. Rising demand for these drugs is the main reason propelling the worldwide glioma market's revenue growth. As the incidence of brain tumours has climbed, so has the need for effective treatment. As a result, international businesses are pouring a sizable portion of their profits into R&D in order to produce cutting-edge drugs.
Market Development
A new, powerful, and selective mutant isocitrate dehydrogenases 1 (mIDH1) inhibitor called safusidenib has demonstrated significant blood-brain barrier permeability. Safusidenib may be a best-in-class treatment for IDH1 mutant lower-grade glioma, according to phase 1 clinical data. For the treatment of recurrent/progressive WHO CNS Grade 2 and Grade 3 IDH1 mutant gliomas, safusidenib is now in Phase II.
High-grade glioma and non-small cell lung cancer are two conditions for which aglatimagene besadenovec is being developed as a therapy. Prostate cancer, advanced non-metastatic pancreatic ductal adenocarcinoma, malignant glioma, paediatric malignant glioma, including glioblastoma multiforme, recurrent glioblastoma multiforme, anaplastic astrocytoma, and recurrent ependymomas.
Chimerix is a biopharmaceutical business on a mission to create drugs that significantly better and prolong the lives of people with life-threatening illnesses. For H3 K27M-mutant glioma, the Company's most advanced clinical-stage development programme, ONC201, is under development.
Market Restraints
Many locals struggle with the big issue of high expenses for anti-cancer treatments. There is intense pressure to reduce costs and demonstrate value. Global political instability and continuous economic stress pose a danger to the sustainability of public healthcare funding. In less developed countries, the lack of accessible drugs has impacted public health and shortened average life expectancy. As a result, pharmaceutical firms are under pressure to reduce global drug prices. Additionally, difficulties with product approval and commercialization, drug side effects, a lack of knowledge and competence about brain cancer and its treatment, and concerns with lack of expertise are all anticipated to significantly hinder market revenue growth.
Key players
Amgen Inc Bristol-Myers Squibb Company Pfizer Inc F. Hoffmann-La Roche Ltd Bayer AG Merck & Co Inc Celgene Corporation Johnson & Johnson AstraZeneca PLC Sanofi1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Canada Glioma Therapeutics Market
By Type of Disease
- Glioblastoma Multiforme
- Anaplastic Astrocytoma
- Anaplastic Oligodendroglioma
- Anaplastic Oligoastrocytoma
- Other
By Therapy
- Chemotherapy
- Targeted Drug Therapy
- Radiation Therapy
- Immunotherapy
By diagnosis
- Neurological exam
- Computed Tomography (CT) scan
- Magnetic Resonance Imaging (MRI)
- Positron Emission Tomography (PET) scan
- Biopsy
- molecular testing
- Electroencephalography (EEG)
By End User
- Hospital
- Clinics
- Ambulatory surgical centres
- Research Center
- Diagnostic Center
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































