Canada Cough Hypersensitivity Syndrome Therapeutics Market Analysis
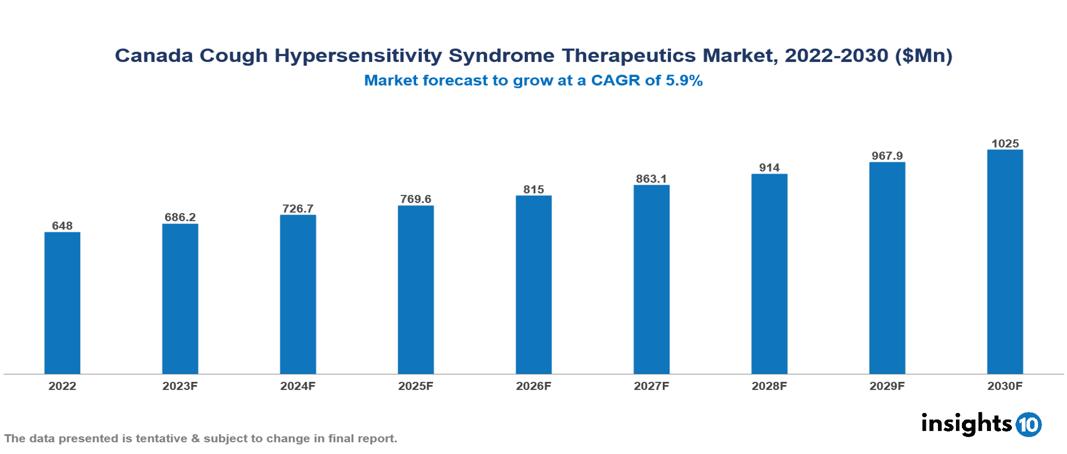
The Canada Cough Hypersensitivity Syndrome Therapeutics Market was valued at $0.648 Bn in 2022 and is predicted to grow at a CAGR of 5.9% from 2023 to 2030, to $1.025 Bn by 2030. The key drivers of this industry include the increasing prevalence of Cough Hypersensitivity Syndrome, growing awareness and diagnosis, and the emergence of advanced therapeutics in the industry. The industry is primarily dominated by players such as Teva, Pfizer, Novartis, Johnson & Johnson, and GSK among others.
Buy Now

Canada Cough Hypersensitivity Syndrome Therapeutics Market Analysis Executive Summary
The Canada Cough Hypersensitivity Syndrome Therapeutics Market is at around $0.648 Bn in 2022 and is projected to reach $1.025 Bn in 2030, exhibiting a CAGR of 5.9% during the forecast period.
Cough Hypersensitivity Syndrome (CHS) is marked by an enhanced response to stimuli, resulting in heightened sensitivity of the cough reflex. This sensitivity results in prolonged coughing caused by irritants such as dust, perfume, or cold air. Common causes of CHS include respiratory infections, gastroesophageal reflux disease (GERD), and exposure to environmental toxins. Symptoms usually include a chronic cough, which may be accompanied by throat irritation or pain. The treatment focuses on addressing the underlying causes and regulating the increased cough reflex. Antitussives, which suppress the cough reflex, and medicines to treat underlying problems like GERD are two possible treatment choices. Companies such as GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim are developing CHS treatments.
Cough Hypersensitivity Syndrome, which causes chronic cough, affects about 16% of Canadians. These estimates are amplified by increased risk factors like increased air pollution, smoking, and others. The market is being driven by significant factors such as the increase in the prevalence of Cough Hypersensitivity Syndrome, growing awareness and diagnosis, and the emergence of advanced therapeutics in the industry. However, conditions such as high costs of treatment, limited treatment options, and stringent regulations limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Rising prevalence of Cough Hypersensitivity Syndrome: CHS frequently occurs as a result of persistent respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and allergic rhinitis. More than 16% of Canadians over the age of 45 suffer from chronic cough. The rising prevalence of these conditions in Canada is directly related to an increased pool of CHS patients requiring effective treatment options. Factors such as air pollution, smoking, and aging populations all contribute to the rise of chronic respiratory disorders, improving the potential for treating conditions like CHS.
Development of advanced therapeutics: CHS treatment is evolving as new drugs and therapies are used to control cough triggers and related mechanisms. This consists of P2X3 receptor antagonists, biologics, and combination therapies, which represent effective treatment alternatives. Pharmaceutical companies are investing significantly in the research and development of medications for CHS, resulting in a pipeline of candidates with the potential to drive market expansion.
Growing awareness and diagnosis: Growing awareness of CHS among healthcare professionals and patients leads to early diagnoses and prompt treatment. This enables timely intervention with therapeutics, resulting in market growth. Public health measures such as the National Lung Health Framework advances in diagnostic techniques, and a better understanding of CHS symptoms contribute to early diagnosis and management of the condition.
Market Restraints
High costs of treatment: Several treatments, such as neuromodulators and P2X3 receptor antagonists, are classified as specialty drugs, which increases expenses for both patients and healthcare systems. This is a challenge for individuals, particularly those with low financial resources or inadequate insurance coverage.
Limited treatment options: Treatment alternatives, like cough suppressants, inhaled corticosteroids, and proton pump inhibitors (PPIs), focus on related problems such as GERD or asthma rather than the cough. Several existing drugs owing to their long-term usage have adverse effects resulting in treatment non-adherence, limiting the market growth
Regulatory hurdles: Developing therapies for CHS faces challenges due to regulatory requirements and a lengthy clinical trial procedure by Health Canada. These factors discourage pharmaceutical companies from investing in R&D for a small patient group.
Notable Updates
April 2023, GSK acquired Canada-based BELLUS Health for a total of $2 Bn. This acquisition gives GSK access to BELLUS's only medication in development, Camlipixant, a P2X3 antagonist that is now in Phase III for the initial treatment of adult patients with refractory chronic cough (RCC)
Healthcare Policies and Regulatory Landscape
In Canada, the principal regulatory body for therapeutics is Health Canada, specifically the Health Products and Food Branch (HPFB). The HPFB regulates pharmaceuticals, biologics, and medical devices to ensure that therapeutic goods available in Canada meet strict safety, efficacy, and quality criteria.
The procedure for gaining therapeutic product licensure in Canada is systematic. Companies have to submit a New Drug Submission (NDS) or a Biologics License Application (BLA), which includes detailed information about the product's efficacy. After completing the regulatory requirements, Health Canada issues a Notice of Compliance (NOC), allowing marketing authorization in Canada
The regulatory framework in Canada requires comprehensive review processes which present barriers for new entrants in the industry
Competitive Landscape
Key Players
- Johnson & Johnson
- Bayer AG
- Teva Canada Limited
- GlaxoSmithKline
- Pfizer
- Boehringer Ingelheim
- AstraZeneca
- F. Hoffman-La Roche Ltd
- Merck
- Novartis
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Cough Hypersensitivity Syndrome Therapeutics Market Segmentation
By Drug Class
- Antitussive Agents
- Corticosteroids
- Short Acting Beta-2 Agonists
- Anti-cholinergic
- Antihistamines
- Proton Pump Inhibitors
- Others
By Route of Administration
- Oral
- Inhalation
- Others
By End Users
- Hospitals
- Speciality Clinics
- Homecare
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.