Canada Clinical Nutrition for Diabetes Care Market Analysis
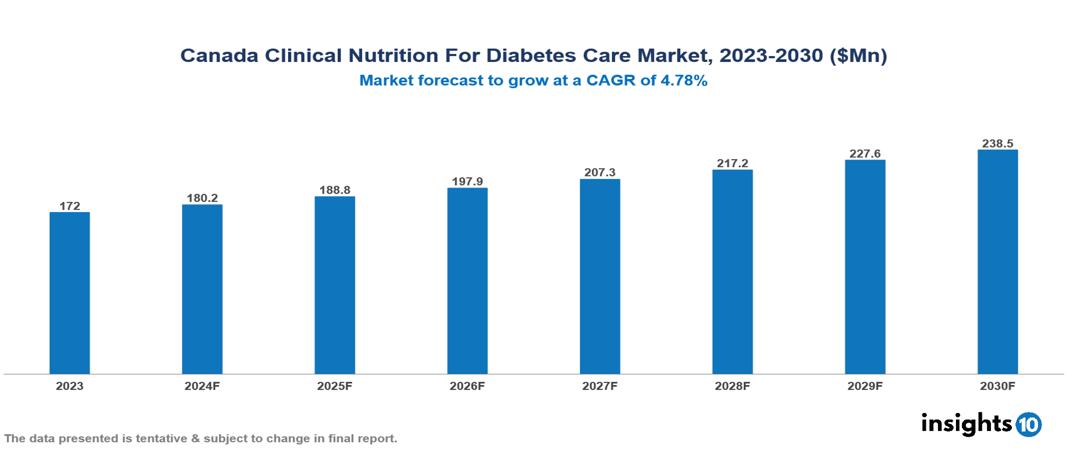
The Canada Clinical Nutrition for Diabetes Care Market was valued at $172 Mn in 2023 and is predicted to grow at a CAGR of 4.78% from 2023 to 2030, to $238.49 Mn by 2030. The key drivers of this industry include increasing the prevalence of diabetes, advancements in nutritional science, and initiatives for diabetes management. The key players in the industry are Abbott Nutrition, Eli Lily, Danone, AgaMatrix Inc., and among others.
Buy Now

Canada Clinical Nutrition for Diabetes Care Market Executive Summary
The Canada Clinical Nutrition for Diabetes Care Market is at around $172 Mn in 2023 and is projected to reach $238.49 Mn in 2030, exhibiting a CAGR of 4.78% during the forecast period.
Clinical nutrition for diabetes care refers to specialized dietary interventions and nutritional strategies designed to manage and improve the health outcomes of people with diabetes mellitus. It involves using evidence-based nutrition treatment to help diabetic patients maintain blood sugar control, avoid complications, and enhance their overall metabolic health. The primary aim is to optimize nutrition, regulate blood sugar levels, prevent complications, and enhance overall health outcomes for those with diabetes.
The increase in the prevalence of diabetes due to aging, obesity, and unhealthy lifestyles is one of the factors contributing to the growth of the global clinical nutrition market. Obesity is a major factor leading to diabetes. Clinical nutrition including medical foods and parenteral nutrition can help relieve symptoms or slow down the progression of a chronic condition. As the number of people with diabetes continues to grow, so does the demand for these specialized food products. Patients afflicted with diabetes are at higher risk of comorbidities, as diabetes is a major cause of blindness, kidney failure, heart attacks, stroke, and lower limb amputation, as reported by the WHO.
The leading pharmaceutical companies include Abbott Nutrition, Eli Lily is known for its extensive portfolio of medications, including those essential for diabetes care. Nestlé Health Science, AgaMatrix Inc., and Sanofi are also significant contributors to the Clinical Nutrition for Diabetes Care landscape, with continuous research and development activities.
Market Dynamics
Market Growth Drivers
Increasing Prevalence of Diabetes: In recent years, diabetes has been on the rise worldwide, primarily due to factors like inactive behaviors, poor dietary choices, and increasing levels of obesity. Canada has a higher diabetes rate in comparison to numerous other nations. According to the International Diabetes Federation, about 7.7% of Canadian adults are living with diabetes. Clinical nutrition plays a crucial role in managing blood sugar and preventing complications.
Rising Geriatric Population: Canada's aging population estimated at around 20% presents a significant opportunity for the clinical nutrition market. Diabetes complications like neuropathy, vision problems, and wounds can be exacerbated by malnutrition. Clinical nutrition programs can help seniors with diabetes maintain optimal nutritional status, potentially slowing the progression of complications and improving overall well-being. Also, a significant portion of the aging population falls into the pre-diabetic category. Clinical nutrition can play a crucial role in preventing the progression to full-blown diabetes by helping individuals adopt healthy eating habits and manage blood sugar levels.
Initiatives for diabetes management: The Canadian Institutes of Health Research (CIHR) and JDRF Canada announced a $30 Mn partnership to jointly fund type 1 diabetes research. After the passage of the National Framework for Diabetes Act in June 2021, the government released the Framework for Diabetes in Canada in October 2022. This framework aims to provide a unified policy direction to improve access to prevention, treatment, and support services for all Canadians with diabetes.
Market Restraints
Stringent Regulations: Strict regulations for introducing new clinical nutrition products can delay market entry and increase research and development costs for companies. Depending on the product's claims and novelty, Health Canada may require extensive clinical trials. These trials can be expensive and time-consuming to conduct, further delaying market entry and increasing R&D costs.
Lack of Access to Registered Dietitians: There is a shortage of healthcare providers specializing in diabetes care and clinical nutrition, especially in rural and remote areas. This shortage can lead to longer wait times for appointments and limited availability of specialized services.
Competition from Mainstream Food and Beverage Products: The availability of mainstream food and beverage products that are marketed as suitable for diabetes management may pose competition to clinical nutrition products, making it challenging for the clinical nutrition market to gain traction.
Regulatory Landscape and Reimbursement Scenario
The regulatory landscape for clinical nutrition products for diabetes care in Canada is overseen by Health Canada, specifically through the Food and Drug Regulations (FDR). Many clinical nutrition products for diabetes care fall under the Foods for Special Dietary Use (FSDU) category. The FSDU regulations define specific compositional and labeling requirements based on the product's intended use. Products must have clear and accurate labeling, including information on ingredients, nutritional content, intended use, and any potential risks or interactions with medications
The Canadian healthcare system generally prioritizes coverage for medical interventions like medications and insulin. Clinical nutrition services and products may not be readily covered by provincial health plans. Some private health insurance plans might offer partial or full coverage for consultations with dietitians or specific clinical nutrition products. However, coverage details vary significantly between plans.
Competitive Landscape
Key Players
Here are some of the major key players in the Canada Clinical Nutrition for Diabetes Care Market:
- Abbott Nutrition
- Pfizer Inc.
- Danone
- Nestlé Health Science
- Dexcom
- Eli Lily
- Sanofi
- AgaMatrix Inc.
- Arkay
- Novo Nordisk
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Clinical Nutrition for Diabetes Care Market Segmentation
By Product
- Oral Nutrition
- Parenteral Nutrition
- Enteral Feedback Formulas
By Stage
- Adult
- Paediatric
By Distribution Channel
- Online
- Retail
- Institutional Sales
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































