Canada Chronic Obstructive Pulmonary Disease (COPD) Therapeutics Market Analysis
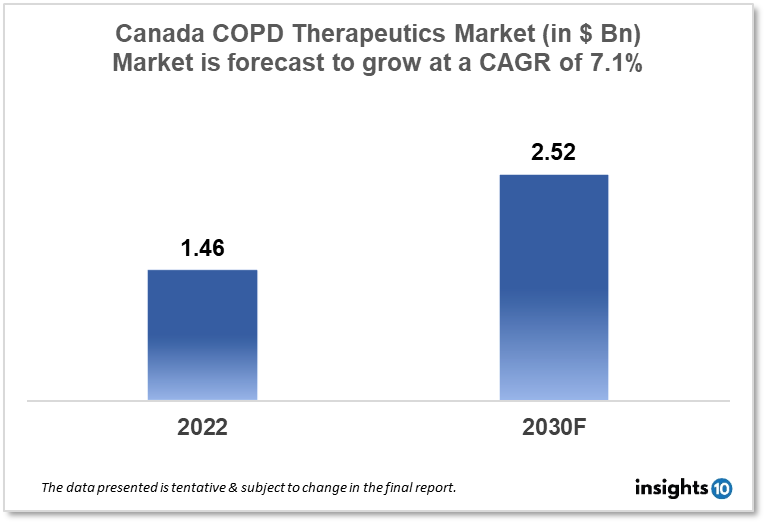
Canada's Chronic Obstructive Pulmonary Disease (COPD) therapeutics market was valued at $1.46 Bn in 2022 and is estimated to expand at a CAGR of 7.1% from 2022 to 2030 and will reach $2.52 Bn in 2030. One of the main reasons propelling the growth of this market is the increased prevalence rate, a result of government initiatives. The market is segmented by Drug class and by distribution channel. Some key players in this market are Abbott Laboratories, Adamis Pharmaceuticals Corporation, Almirall, Astellas Pharma, AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Novartis, Pfizer, Teva Canada Limited, and others.
Buy Now

Canada Chronic Obstructive Pulmonary Disease (COPD) Therapeutics Market Executive Summary
Canada's COPD therapeutics market was valued at $1.46 Bn in 2022 and is estimated to expand at a CAGR of 7.1% from 2022-30 and reach $2.52 Bn in 2030. Chronic obstructive pulmonary disease (COPD) is a chronic condition characterized by shortness of breath, cough, and sputum production. While signs of the disease do not commonly develop in those under the age of 55, lung alterations begin many years earlier. COPD is a catch-all term for a variety of respiratory disorders, including chronic bronchitis and emphysema. COPD deteriorates gradually over time. More frequent exacerbations, additional reductions in airflow, and earlier death are all connected with increasing disease severity. Shortness of breath limits people's activity levels and lowers their quality of life as the disease progresses.
Many people take their breathing for granted. In Canada, however, 3.8 Mn people over the age of one have asthma, and 2.0 Mn have chronic obstructive pulmonary disease (COPD), both of which can impair a person's capacity to breathe. People suffering from asthma or COPD may find it difficult to participate in daily life, school, work, and social activities. There is also a collective consequence in terms of missed productivity and healthcare expenses, especially given the rising prevalence of both asthma and COPD.
Market Dynamics
Market Growth Drivers
COPD is a major health issue in Canada, with a significant portion of the population affected by the disease. According to the Lung Association, an estimated 3 Mn Canadians are living with COPD, and the disease is responsible for approximately 10% of all deaths in the country. This growing prevalence of COPD is expected to drive the demand for COPD therapeutics in Canada. Combination therapy with bronchodilators and corticosteroids is often used to improve symptom control and reduce exacerbations in patients with COPD. The global COPD therapeutics market is expected to be driven by the increasing demand for combination therapies. This trend is expected to be reflected in the Canada market as well. The introduction of new drugs for the treatment of COPD is also expected to drive the growth of the COPD therapeutics market in Canada. For example, in 2019, Health Canada approved Trelegy Ellipta, a triple combination therapy for the treatment of COPD. The approval of new drugs is expected to expand the options for patients and improve outcomes. The Canadian government has also taken initiatives to address the growing burden of COPD. For example, the Canadian Thoracic Society has developed guidelines for the management of COPD, and the government has implemented policies aimed at reducing smoking rates, which is a major risk factor for COPD. These initiatives are expected to increase awareness of COPD and drive demand for COPD therapeutics in Canada.
Market Restraints
The cost of COPD treatment in Canada can be quite high, particularly for patients who require combination therapy or newer medications. This can make it difficult for some patients to access the treatment they need, particularly those who do not have adequate health insurance coverage. COPD is a chronic disease that requires long-term management, and adherence to treatment can be a challenge for some patients. This can be due to a variety of factors, including forgetfulness, difficulty using inhalers, or side effects from medication. Poor adherence to treatment can lead to worsened symptoms, increased risk of exacerbations, and poorer outcomes. Several key COPD medications have already lost patent protection or are set to lose patent protection in the coming years. This is expected to lead to increased competition from generic drugs, which could put pressure on prices and margins for manufacturers of branded medications. The regulatory environment for pharmaceuticals in Canada can be challenging, with lengthy approval processes and strict requirements for clinical trials. This can make it difficult for new drugs to enter the market, particularly for smaller companies with limited resources. Despite the availability of various medications for COPD, some patients may not respond well to current treatments or may experience side effects that limit their use. This highlights the need for continued research and development of new therapies for COPD.
Competitive Landscape
Key Players
- Abbott Laboratories
- Adamis Pharmaceuticals Corporation
- Almirall,
- Astellas Pharma
- AstraZeneca
- Boehringer Ingelheim Pharmaceuticals
- Novartis
- Pfizer
- Teva Canada Limited
Healthcare Policies and Regulatory Landscape
Health Canada is the federal department responsible for overseeing the safety and effectiveness of pharmaceuticals and medical devices in Canada. All COPD medications must be approved by Health Canada before they can be marketed in the country. Health Canada also monitors the safety of drugs after they are on the market and can act if safety concerns arise.
Patented Medicine Prices Review Board (PMPRB) is an independent agency that regulates the prices of patented drugs in Canada. The PMPRB sets maximum prices that pharmaceutical companies can charge for their drugs, based on the value of the drug and the prices in other countries. The PMPRB also monitors the prices of drugs over time and can adjust the maximum price if necessary. Each Canadian province and territory have its own drug plan that provides coverage for prescription drugs. The drug plans vary by province and may cover different drugs, have different co-payments, and have different eligibility criteria. Pharmaceutical companies must negotiate with each province separately to have their drugs covered under the provincial drug plans.
Canadian Agency for Drugs and Technologies in Health (CADTH) is an independent agency that provides evidence-based information about the effectiveness and cost-effectiveness of drugs and medical devices. CADTH evaluates drugs and makes recommendations to the provincial and territorial drug plans about whether the drugs should be covered and at what price.
Canadian Institutes of Health Research (CIHR) is the federal agency responsible for funding health research in Canada. CIHR provides funding for research related to COPD, including research into new treatments and therapies. This research can inform the development of new drugs and improve patient outcomes.
Overall, the healthcare policy and regulatory framework in Canada plays a critical role in shaping the Canada Chronic Obstructive Pulmonary Disease (COPD) Therapeutics Market. The policies and regulations aim to balance patient access to effective treatments with the need to control healthcare costs and promote safety and effectiveness.
Reimbursement Scenario
In Canada, healthcare is publicly funded and available to all citizens and permanent residents. However, some individuals may have private health insurance through their employer or purchase it independently. Private insurance plans may have different reimbursement policies and may not cover all of the same drugs as public drug plans. Many provincial drug plans require patients to pay a portion of the cost of their prescription drugs, known as a co-payment. The co-payment amount varies by province and may be based on income or other factors. Some provincial drug plans have tiered formularies, which categorize drugs into different tiers based on their cost and effectiveness. Drugs in higher tiers may have higher copayments or may not be covered at all.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Chronic Obstructive Pulmonary Disease (COPD) Therapeutics Market Segmentation
By Drug Class
- Bronchodilators: Bronchodilators are medications that help to relax the muscles around the airways, making it easier to breathe. These can be further classified as short-acting or long-acting bronchodilators
- Corticosteroids: Corticosteroids are anti-inflammatory medications that can help reduce swelling and inflammation in the airways. These can be used alone or in combination with bronchodilators
- Combination therapies: Combination therapies combine bronchodilators and corticosteroids in a single medication. These are often used for patients with more severe COPD
- Phosphodiesterase-4 inhibitors: Phosphodiesterase-4 inhibitors are medications that help to reduce inflammation and improve airflow in the lungs
- Others: Other medications that may be used to treat COPD include mucolytics, oxygen therapy, and vaccines for influenza and pneumococcal disease
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































