Canada Blood Group Typing Market Analysis
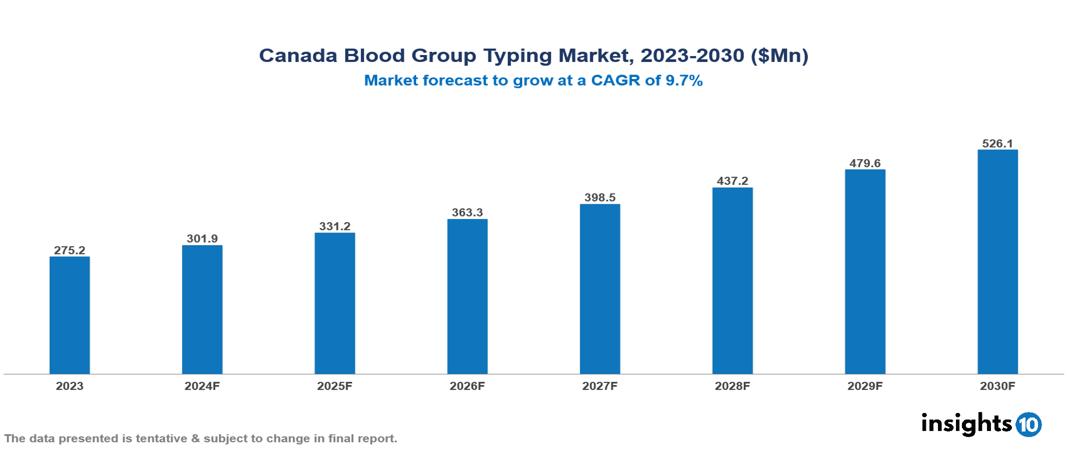
The Canada Blood Group Typing Market was valued at $275.20 Mn in 2023 and is predicted to grow at a CAGR of 9.70% from 2023 to 2030, to $526.13 Mn by 2030. The key drivers of this industry are increase in prevalence of chronic diseases, public initiatives, and rising number of surgical procedures. The key players in the industry are Grifols Canada, BAG Diagnostics GmbH, Roche Diagnostics, and Abbott Laboratories.
Buy Now

Canada Blood Group Typing Market Executive Summary
The Canada Blood Group Typing Market is at around $275.20 Mn in 2023 and is projected to reach $526.13 Mn in 2030, exhibiting a CAGR of 9.70% during the forecast period.
Blood typing is a method to tell what type of blood the individual has. Blood typing is done so they can safely donate or receive a blood transfusion. Blood group determination helps understand a person’s blood group and determine the presence of Rh factor in RBCs. ABO and Rh blood group typing procedures are used to determine an individual’s blood type, which is essential for safe blood transfusions. The presence or lack of specific proteins on RBCs known as antigens shape a person’s blood type, which is inherited from their parents. Four main blood types are distinguished using the ABO blood typing system: Type A, Type B, Type AB, and Type O.
Blood typing is important for several reasons like, safe blood transfusions and matching donor and recipient blood types prevent potentially life-threatening transfusion reactions. Pregnant women must know their blood type, especially the Rh factor. This helps prevent complications like hemolytic disease in the newborn. Blood typing can provide information for genetic studies and inheritance patterns. It also plays a key factor in organ transplant matching to reduce the risk of organ rejection.
The increase in donations over the years signifies that the number of blood group typing procedures has also increased. Furthermore, the increasing prevalence of diseases associated with blood, such as sickle cell anemia, thalassemia, lymphoma, leukemia, and haemophilia, is also supporting the growth of blood group typing. Approximately 5,000 Canadians live with sickle cell disease. The market therefore is driven by significant factors like the increase in prevalence of chronic diseases, public initiatives, and rising number of surgical procedures. However, cost constraints, geographical challenges, and regulatory challenges restrict the growth of the market.
The prominent companies for blood group typing include Grifols Canada and Bio-Rad Laboratories. Beckman Coulter, Quotient Limited, and Immucor, Inc. are also significant contributors to Canada Blood Group Typing Market.
Market Dynamics
Market Growth Drivers
Increasing Prevalence of Chronic Diseases: The rise in chronic diseases, such as cancer and cardiovascular conditions, necessitates more frequent blood typing for effective treatment and management. More than 1.5 million Canadians (nearly 4% of the population) are living with cancer. This is contributing to the growing demand for blood typing services in clinical settings
Public Initiatives: Increased public awareness regarding the importance of blood donation and typing by Canadian Blood Services is leading to higher participation in blood donation drives. This is crucial for maintaining adequate blood supplies and ensuring that blood typing services can meet demand
Rising Number of Surgical Procedures: As the frequency of surgical procedures increases, so does the need for blood typing to ensure compatibility for transfusions. The growing number of elective and emergency surgeries directly drives the demand for blood typing services.
Market Restraints
Cost Constraint: Adopting advanced blood typing methods like polymerase chain reaction (PCR) and next-generation sequencing (NGS) can be expensive, especially for smaller healthcare facilities and blood banks. The significant expenses involved in acquiring and maintaining sophisticated equipment restrict access to these technologies, particularly in settings with limited resources.
Geographical Challenge: Canada's vast size makes it challenging to collect, transport, and deliver blood promptly, especially in remote areas. Blood collection centers are often concentrated in urban areas, leaving remote communities with limited access to blood services. Timely delivery of blood is essential, but perishable blood products can expire during transport, especially in remote areas.
Regulatory Challenge: The blood typing market is subject to stringent regulatory standards that govern blood transfusions and testing procedures. Compliance with these regulations requires substantial investments in training, quality control, and technology, which can be challenging for smaller organizations to sustain. The complexity of maintaining compliance may deter some facilities from adopting advanced blood typing methods
Regulatory Landscape and Reimbursement Scenario
Health Canada is the primary regulatory body overseeing the safety of the blood supply in Canada. It establishes regulations that blood operators must follow to ensure the safety and quality of blood and blood components for transfusion and further manufacturing. Canadian Standards Association (CSA) Blood Standard is referenced in the Blood Regulations and outlines safety, efficacy, and quality requirements for blood collection, processing, and transfusion. Compliance with this standard is mandatory for blood operators.
Blood products, including those related to blood typing, are typically funded by provincial health ministries. Hospitals do not directly purchase blood products; instead, they receive them at no charge from CBS or Héma-Québec.
Competitive Landscape
Key Players
Here are some of the major key players in the Canada Blood Group Typing Market:
- Grifols Canada
- Bio-Rad Laboratories
- Ortho Clinical Diagnostics
- Beckman Coulter
- Roche Diagnostics
- Quotient Limited
- Immucor, Inc.
- Abbott Laboratories
- Diasorin
- BAG Diagnostics GmbH
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Blood Group Typing Market Segmentation
By Product
- Consumables
- Instruments
- Services
By Techniques
- PCR-based and Microarray Techniques
- Assay-based Techniques
- Massively Parallel Sequencing Techniques
- Other Techniques
By Test Type
- Antibody Screening
- HLA Typing
- Cross-matching Tests
- ABO Blood Tests
- Antigen Typing
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































