Canada Advanced Therapy Medicinal Products (ATMPs) Market Analysis
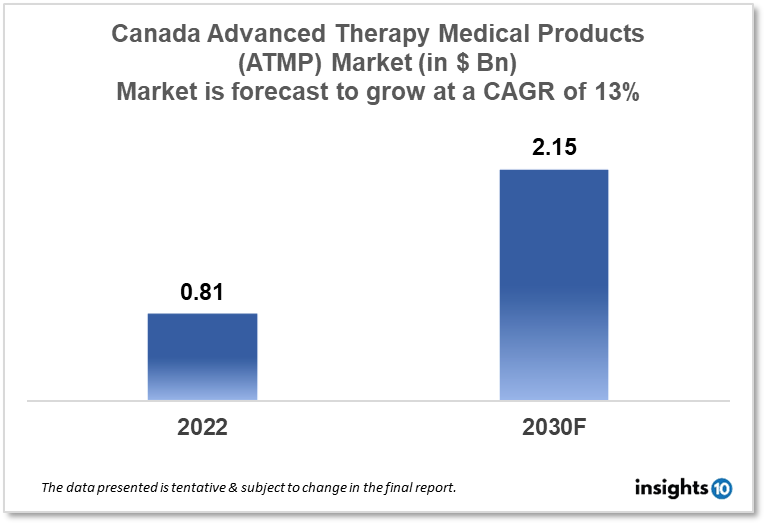
Canada's Advanced Therapy Medicinal Products (ATMPs) market is likely to grow at a CAGR of 13% from a market size of $0.81 Bn in 2022 to $2.15 Bn in 2030. The major growth drivers driving the market are the increasing prevalence of chronic diseases and the growing investment in the industry. This report is segmented by product type, disease type, and by distribution channel. Some key players in this market include Novartis, Celgene Corporation, Gilead Lifesciences, Bluebird Bio, and others.
Buy Now

Canada Advanced Therapy Medicinal Products (ATMPs) Market Executive Summary
Canada's Advanced Therapy Medicinal Products (ATMPs) Market size is at around $0.81 Bn in 2022 and is projected to reach $2.15 Bn in 2030, exhibiting a CAGR of 13% during the forecast period 2022-2030. One of the largest nations in the globe and the largest in the western hemisphere is Canada. With firms ranging from little owner-managed businesses to multinational corporations, it boasts a thriving free-market economy. In the past, the export of agricultural staples, primarily grain, as well as the production and sale of natural resource exports, such as minerals, oil and gas, and forest products, were the foundations of Canada's economy.
The public sector finances healthcare services and the private sector provides these services, in Canada's hybrid public-private healthcare system. There is currently no national procurement model that is uniform. Each province like British Columbia, Ontario, Quebec, and others has its own set of regulations, which can vary significantly between regions or service providers. Provincial governments are implementing or looking at healthcare procurement reforms, including centralized approaches. The research, development, production, and distribution of medications are all covered by the pharmaceutical industry. Global pharmaceutical sales hit $1.42 trillion in 2021 as a result of the market's explosive growth during the previous 20 years. The manufacturing sector has seen a substantial transformation due to the advent of new technologies as well as more cost-effective and efficient manufacturing methods. Moreover, increasing investment flow in this industry has favorably influenced market growth.
Advanced Treatment Medicinal Products (ATMPs) have the ability to treat the underlying cause of disease as opposed to just treating the symptoms. ATMPs help give revolutionary advantages that are not provided by traditional therapy. These elements are anticipated to drive the market during the forecast period.
Market Dynamics
Market Growth Drivers
In Canada, chronic diseases like cancer, diabetes, and heart disease are major causes of concern. In the upcoming years, demand for ATMPs may increase as a result of their potential to treat various disorders with focused and individualized therapies. The Canadian government has taken initiatives to promote the growth of the ATMP sector. This includes legislative frameworks that permit accelerated approval of these goods as well as funding schemes for ATMP research and development. Technology advancements like CRISPR gene editing and 3D printing are spurring innovation in the ATMP sector. These developments are making it possible to create more sophisticated and successful treatments, which could be very advantageous for patients. Venture capital firms have made large investments in the ATMP sector. This investment is promoting the creation of new goods and technology and expands the funding sources for the development of ATMPs. In Canada, several ATMP products are now being developed, including tissue engineering goods for wound healing, cell therapies for cancer, and gene therapies for uncommon disorders. The demand for ATMPs may increase as a result of these products' ability to solve important unmet medical needs in the years to come.
Market Restraints
ATMP development can be expensive, especially for small businesses and startups. For businesses wishing to enter the ATMP sector, these high prices can be a substantial barrier, which may reduce industry competitiveness and innovation. ATMP manufacture calls for specific equipment and facilities, which can be costly to create and maintain. Due to production constraints caused by this restricted manufacturing capacity, the marketability of ATMPs may be constrained. ATMPs can be expensive to construct and manage, which makes reimbursement difficult. Also, the reimbursement procedure for these items could not be well understood, which might prevent widespread market acceptance.
Competitive Landscape
Key Players
- Novartis
- Celgene Corporation
- Gilead Lifesciences
- Bluebird Bio
- Spark Therapeutics
- Kolon TissueGene
Healthcare Policies and Regulatory Landscape
Gene treatments, cell therapies, and tissue-engineered products all fall under the category of advanced therapy medicinal products (ATMPs). Health Canada, the nation's regulatory body for pharmaceuticals and medical devices, oversees ATMP regulation in Canada. To guarantee the security, efficiency, and calibre of ATMPs, Health Canada has devised a regulatory framework. This framework features an accelerated assessment and approval process for ATMPs, allowing for streamlined regulatory oversight of these goods. ATMPs need to adhere to the same regulatory standards as other medications and biologics in order to be used in Canada. This entails adhering to Good Manufacturing Procedures (GMP) for the development and testing of the product, as well as preclinical and clinical testing to determine its safety and effectiveness. Companies creating ATMPs can also get advice from Health Canada, including how to create gene treatments, cell therapies, and tissue-engineered goods. This advice provides suggestions for clinical trial layout as well as instructions for the production and quality assurance of these items. There are only a few ATMPs that have been authorized for use in Canada as of yet. Nonetheless, a variety of ATMP products are being developed in Canada, including tissue-engineered products for wound healing, cell therapies for cancer, and gene therapies for uncommon disorders.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Advanced Therapy Medicinal Products (ATMPs) Market Segmentation
By Product Type (Revenue, USD Billion):
- Cell Therapy
- Gene Therapy
- CAR-T Therapy
- Tissue Engineered Product
By Disease Type (Revenue, USD Billion):
- Alzheimer's
- Cystic Fibrosis
- Muscular Dystrophies
- Hemophilia
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacy
- Drug Store
- Retail Store
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































