Canada Acute Lymphocytic Leukemia (ALL) Therapeutics Market Analysis
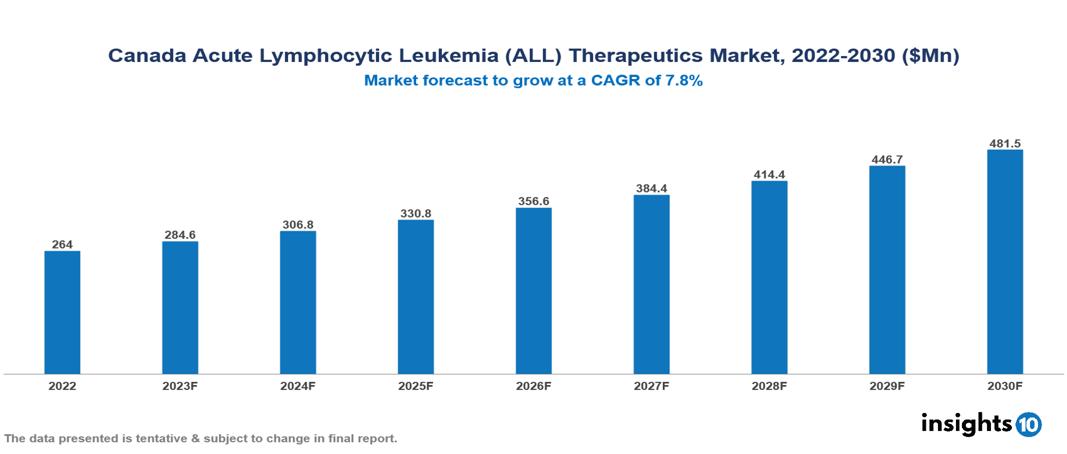
The Canada Acute Lymphocytic Leukemia (ALL) Therapeutics Market was valued at US $264 Mn in 2022 and is predicted to grow at a CAGR of 7.8% from 2023 to 2030, to US $481 Mn by 2030. The key drivers of this industry include the upward trend in the incidence of acute lymphocytic leukaemia cases, advancements in molecular biology and pharmacology technologies, and other factors. The industry is primarily dominated by players such as Pfizer, Sanofi, Novartis, Genmab among others.
Buy Now

Canada Acute Lymphocytic Leukemia (ALL) Therapeutics Market Analysis: Executive Summary
The Canada Acute Lymphocytic Leukemia (ALL) Therapeutics Market is at around US $264 Mn in 2022 and is projected to reach US $481 Mn in 2030, exhibiting a CAGR of 7.8% during the forecast period.
Acute lymphocytic leukemia (ALL) is a type of cancer that develops in the blood and bone marrow, targeting white blood cells known as lymphocytes. It is the most prevalent type of cancer in children and can also occur in adults; however, the chances of a cure are limited. The disease spreads rapidly and, if not treated, results in fatalities within a few months. Common symptoms include frequent infections, lymph node enlargement, weight loss, and bone pain, among others. Treating ALL requires a comprehensive, prolonged approach involving chemotherapy, targeted therapy, CAR-T cell immunotherapy, and, in extreme cases, stem cell transplantation. These advancements have notably improved cure rates, reaching up to 80% in children and young adults.
ALL is the most commonly diagnosed childhood cancer in Canada. Recently, it is reported that 385 Canadians were diagnosed with ALL, out of which 220 were males and 165 were females, indicating an increase in the disease prevalence. The market is therefore driven by major factors like rapid product developments and approvals, increased investment by governmental agencies, and consumer knowledge of the therapeutics industry. However, conditions such as adverse side effects of several drugs and drug shortages in response to demand hinder the growth and potential of the market.
One of the notable players in the market is Bristol-Myers Squibb, which has recently obtained approval for the drug Breyanzi, a CAR T cell therapy to treat adult patients with B-cell ALL, from the US FDA.
Market Dynamics
Market Growth Drivers
Rise in the incidence of ALL: In Canada, childhood leukemia, including ALL, is the most prevalent cancer in children. The median age of diagnosis is 8 years in Canada, with the peak incidence of ALL in the age group of children between 0 and 4 years.
New product development and approval: The rapid progression in leukemia and new products are expected to propel this sector forward in the forecasted years. The approval of drugs like Kymriah by Novartis AG in different geographic regions is further driving the growth.
Increased awareness: The Canadian market for acute lymphocytic leukemia treatments is seeing growth due to the proactive efforts of both governmental and private entities. This resulted in increased consumer knowledge about cancer drugs and therapies including ALL which shows an upward trend in the growth of the market.
Market Restraints
Side effects associated with treatments: Chemotherapy is expected to dominate the market in the forecasted year and the side effects associated with this treatment can hamper and limit the expansion of this industry.
Drug Shortage: Shortage of drugs during the forecasted period is estimated to be a hindrance in the growth of the ALL-therapeutics market which wis caused by factors relating to manufacturing, supply chin inefficiency and increased demand.
Notable Recent Updates
May 2, 2023, Breyanzi (lisocabtagene maraleucel, liso-cel) underwent testing within the pivotal TRANSCEND CLL Phase 1/2, 004 study. This open-label, single-arm multicenter study assessed its efficacy in adults experiencing relapsed or resistant chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
November 17, 2022, Takeda announced that the Phase 3 randomized PhALLCON trial had achieved its primary endpoint. The results showed that ICLUSIG (ponatinib) in conjunction with reduced-intensity chemotherapy led to a higher percentage of minimal residual disease (MRD)-negative complete remission (CR) in older individuals with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) than imatinib.
Healthcare Policies and Regulatory Landscape
The Canada Health Act (CHA), outlines the key objectives of Canadian healthcare policy and regulates the country's publicly funded healthcare system. The policy aims "to protect, promote, and restore the physical and mental well-being of residents of Canada and to facilitate reasonable access to health services without direct charges at the point of service for such services."
The CHA set down standards that the territories and provinces must meet in order to be eligible for federal financing, both for extended health care services and covered health services.
Obtaining a medical license in Canada involves a province- and territory-specific process mandated by Health Canada (HC), typically necessitating the completion of a medical degree, residency training, and successful passage of licensing examinations.
Competitive Landscape
Key Players
- Pfizer Inc.
- Bristol-Myers Squibb Company
- Novartis AG
- SymBio Pharmaceuticals Limited
- F. Hoffman-La-Roche Ltd
- Erytech Pharma
- Sanofi
- Takeda Pharmaceuticals, Inc
- Spectrum Pharmaceuticals, Inc
- Amgen, Inc
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Canada Acute Lymphocytic Leukemia (ALL) Therapeutics Market Segmentation
By Type
- Paediatrics
- Adults
By Drug
- Hyper CVAD regimen
- Linker Regimen
- Nucleoside Metabolic Inhibitors
- Targeted drugs and Immunotherapy
- CALGB 811 Regimen
By Cell
- B Cell ALL
- T Cell ALL
- Philadelphia Chromosome
By Therapy
- Chemotherapy
- Targeted therapy
- Radiation therapy
- Stem Cell Transplantation
By Distribution channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.