Brazil Oncology Therapeutics Market Analysis
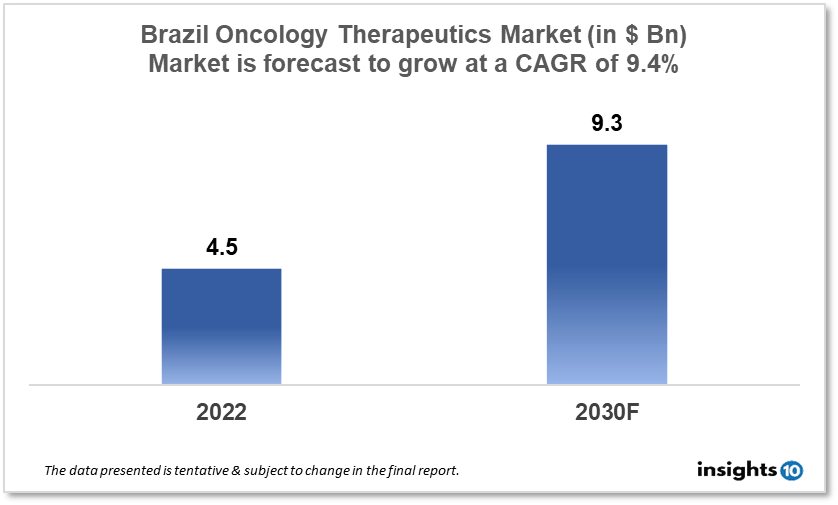
By 2030, it is anticipated that the Brazil Oncology Therapeutics Market will reach a value of $9.3 Bn from $4.5 Bn in 2022, growing at a CAGR of 9.4% during 2022-2030. The Oncology Therapeutics Market in Brazil is dominated by a few domestic pharmaceutical companies such as Cristalia, Eurofarma, and Ache Laboratorios. The Oncology Therapeutics Market in Brazil is segmented into different types of cancer and different therapy type. The major risk factors associated with cancer are diet, alcohol, tobacco, air pollution, and physical inactivity. The demand for Brazil Oncology Therapeutics is increasing on account of the rise in initiatives taken by the Government of the country.
Buy Now

Brazil Oncology Therapeutics Market Analysis Summary
By 2030, it is anticipated that the Brazil Oncology Therapeutics Market will reach a value of $9.3 Bn from $4.5 Bn in 2022, growing at a CAGR of 9.4% during 2022-30.
Brazil is an upper middle-income, developing and the largest country in South America bounded by the Atlantic Ocean. In Brazil, it was anticipated that 600,000 new cancer cases would be diagnosed each year in 2018 and 2019. Cancer care in Brazil was substantially absorbed by philanthropic groups from the early twentieth century until 1937. The National Cancer Institute was established that year, with a distinct role in policymaking, but the status quo of care remained unchanged.
High-complexity oncology centres (Centros de Assistência de Alta Complexidade em Oncologia-CACONs), high-complexity oncology units (Unidades de Assistência de Alta Complexidade em Oncologia-UNACONs), and hospital complexes provide cancer treatment. Brazil's government spends 10.3 % of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers
Radiotherapy is crucial since nearly half of all cancer patients receive it at some point throughout their treatment. The "Plan for Radiation Therapy Expansion in the SUS" was formed in 2012 to reduce the treatment coverage gap in Brazil by constructing or expanding health services. In contrast to the previous year, the sale of cancer pharmaceuticals climbed by an average of 30% between February 2021 and January 2022, indicating that the oncology drugs market in Brazil is progressing well. These aspects could boost Brazil's Oncology Therapeutics Market.
Market Restraints
In Brazil, access to radiotherapy facilities is a societal concern, with over 100,000 people dying each year owing to a lack of proper radiation therapy. The healthcare system in Brazil is fragmented, with both public and private providers coexisting. This may make obtaining and administering lung cancer drugs challenging for patients and physicians. Human-use drugs face one of the largest tax burdens in Brazil, amounting to an average of 31.3 % of the consumer price, preventing the items from being supplied at even lower costs to consumers. Private insurance accounts for around 57 % of health spending, with out-of-pocket expenses, especially for pharmaceuticals, accounting for more than half of that amount. Brazil's competitiveness is hampered by high manufacturing costs. These factors may deter new entrants into the Brazil Oncology Therapeutics Market.
Competitive Landscape
Key Players
- Ache Laboratorios Farmaceuticos: Ache Laboratorios Farmaceuticos is a Brazilian pharmaceutical company that produces a range of cancer therapeutics, including chemotherapy drugs, targeted therapies, and biosimilars
- Cristalia Produtos Quimicos Farmaceuticos: Cristalia Produtos Quimicos Farmaceuticos is a Brazilian pharmaceutical company that produces a range of cancer therapeutics, including chemotherapy drugs, targeted therapies, and biologics. The company has a strong focus on research and development and has several innovative cancer drugs that are currently in clinical development
- Eurofarma Laboratorios: Eurofarma Laboratorios is a Brazilian pharmaceutical company that produces a range of cancer therapeutics, including chemotherapy drugs, targeted therapies, and biosimilars. The company has a strong presence in Brazil and has expanded its operations to other countries in Latin America
- Blau Farmaceutica: Blau Farmaceutica is a Brazilian pharmaceutical company that produces a range of cancer therapeutics, including chemotherapy drugs, targeted therapies, and biosimilars
Notable Recent Deals
July 2022: The precision oncology diagnostic lab based on next-generation sequencing will allow Hospital de Base in So José do Rio Preto, Brazil, to provide in-house clinical testing to patients in the area while also advancing clinical research. Geneseeq, which has offices in Toronto and Nanjing, will help the Brazilian hospital construct a variety of genomic profiling assays, including its comprehensive pan-cancer gene panels and various cancer type-specific gene panels, under the conditions of the deal.
May 2022: Eurofarma, one of Brazil's most inventive pharmaceutical companies, has established a partnership with Shanghai Henlius Biotech, Inc., a Chinese pharmaceutical company specialising in biologic pharmaceuticals, for the production and sale of three biosimilars throughout Latin America. The new licencing agreement between the companies will allow Eurofarma to expand its cancer medication line in the countries where it operates, bringing more treatment options to the local market.
Healthcare Policies and Reimbursement Scenarios
In Brazil, the administrative body liable for the endorsement and guidelines of helpful items, including disease therapeutics, is the Brazilian Health Regulatory Agency (ANVISA). In the public medical care framework, the expense of malignant growth therapies, including disease therapeutics, is covered by the Brazilian Unified Health System (SUS). The SUS is a public program that gives free medical services administrations to Brazilian residents and qualified occupants.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Oncology Therapeutics Segmentation
By Application (Revenue, USD Billion):
- Blood Cancer
- ?Colorectal Cancer
- Gastrointestinal Cancer
- Gynaecologic Cancer
- Breast Cancer
- Lung Cancer
- Prostate Cancer
- ?Others
By Drugs (Revenue, USD Billion):
- Revlimid
- Avastin
- Herceptin
- Rituxan
- Opdivo
- Gleevec
- Velcade
- Imbruvica
- Ibrance
- Zytiga
- Alimta
- Xtandi
- Tarceva
- Perjeta
- Temodar
- Others
By Therapy (Revenue, USD Billion):
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Hormonal Therapy
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Parenteral
- Others
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- ?Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
By 2030, it is anticipated that the Brazil Oncology Therapeutics market will reach a value of $9.3 Bn from $4.5 Bn in 2022, growing at a CAGR of 9.4% during 2022-2030.
In Brazil, the administrative body liable for the endorsement and guidelines of helpful items, including disease therapeutics, is the Brazilian Health Regulatory Agency (ANVISA).
The Oncology Therapeutics Market in Brazil is dominated by a few domestic pharmaceutical companies such as Cristalia, Eurofarma, and Ache Laboratorios.








































