Brazil Neurology Devices Market Analysis
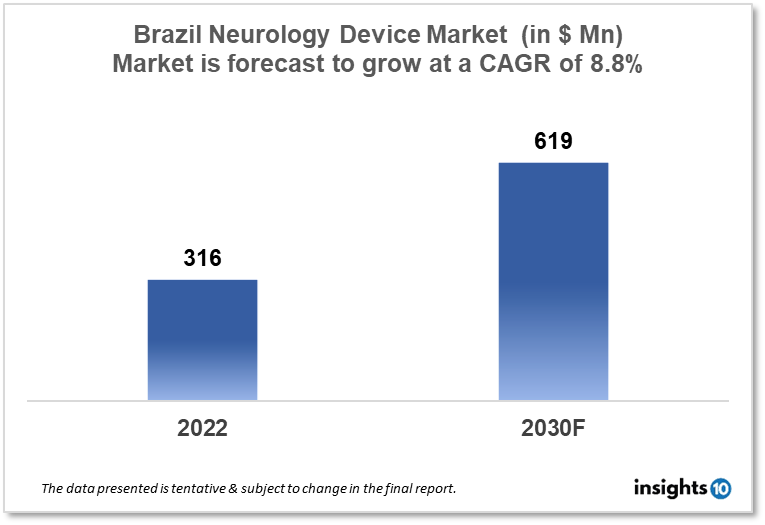
Brazil's Neurology Device Market size is at around $316 Mn in 2022 and is projected to reach $619 Mn in 2030, exhibiting a CAGR of 8.8% during the forecast period. Brazil has a high incidence of neurological disorders such as epilepsy, Parkinson's disease, and migraine, which is driving demand for neurology devices that is captured by both local and international players like Neuroservice, Neurosoft, NeuroRx, Medtronic, and Boston Scientific. This report by Insights10 is segmented by product type like neurostimulation, interventional neurology, neurosurgery devices, and neuro-endoscopes, and by the end user.
Buy Now

Brazil Neurology Device Market Executive Summary
Brazil's Neurology Device Market size is at around $316 Mn in 2022 and is projected to reach $619 Mn in 2030, exhibiting a CAGR of 8.8% during the forecast period. Brazil has distinct characteristics. In some ways, it is typical of a third-world country (dear reader, please feel free to call it a developing country). Because of poor cleanliness, inadequate accommodation, and challenging access to clean water, our neurology wards and outpatient clinics are still overcrowded. Limited access to technology during high-risk prenatal care and labor, as well as a lack of even basic screening for widespread diseases, is a continual impediment to neurodevelopmental disorder prevention. Cerebral palsy, Chagas disease, and occasionally neuro-schistosomiasis are all still common illnesses caused by such an environment. Even neuro-syphilis, along with the most recent group of younger patients, should not be overlooked as a developing problem among the current sexually transmissible illnesses.
Several social and economic indices have improved gradually and significantly over the last few decades, including an increase in life expectancy, which is especially noticeable in some regions, such as the southeast, which has a generally higher quality of life than the north and northeast. The prevalence of dementia is rapidly increasing in the most affluent areas. The same thing is happening with vascular and degenerative illnesses, thanks in part to an increasingly sedentary and obese population, which is increasing the prevalence of stroke and multi-infarct dementia, which is now frequently encountered in conjunction with Alzheimer's disease. However, it is not uncommon to see dementia caused by vitamin deficiencies, revealing the still and always existent starvation diet in some of our people, even in urban areas. Brazilian medical practitioners must be prepared to deal with the frequent designation of a "tropical disorder," now an apparent misnomer, along with prevalent neurodegenerative disorders associated with population aging and general improvements in basic treatment that may help one live longer, but not necessarily better. This corresponds well with the current social and economic disparity that is overwhelming the Brazilian population; undoubtedly, closing this gap is now the top priority.
Some of the most prevalent neurological diseases in Brazil are:
- Stroke
- Alzheimer’s Disease
- Parkinson’s Disease
- Epilepsy
- Multiple sclerosis (MS)
Brazil has one of the highest rates of stroke in the world, due to high levels of obesity, hypertension, and diabetes. For aneurysms, the most common neurological device used is an endovascular coil, which is a small wire mesh that is inserted into the aneurysm to block blood flow. Brazil has a growing elderly population, and Alzheimer's disease is becoming increasingly common in the country. There are currently no neurological devices specifically designed for Alzheimer's disease. However, some researchers are exploring the use of implantable devices that could stimulate the brain to improve memory and cognitive function. Brazil has a high incidence of Parkinson's disease, particularly among elderly individuals. Deep Brain Stimulation (DBS) is used, which involves the implantation of electrodes into specific regions of the brain and the use of electrical stimulation to regulate brain activity. Epilepsy is a common neurological condition in Brazil, affecting both children and adults. MS is a growing problem in Brazil, particularly in urban areas, where the incidence of MS has increased in recent years, there are various assistive devices and technologies that can help manage the symptoms of MS, such as mobility aids, communication devices, and assistive technologies for daily living.
Market Dynamics
Market Growth Drivers
There are several factors contributing to the growth of the neurology device market in Brazil, like:
- Increasing incidence of neurological disorders: Brazil has a high incidence of neurological disorders such as epilepsy, Parkinson's disease, and migraine, which is driving demand for neurology devices
- Growing aging population: The aging population in Brazil is increasing, which is leading to a higher prevalence of age-related neurological conditions and a growing demand for neurology devices
- Improved healthcare infrastructure: The Brazilian government has been investing in improving healthcare infrastructure and increasing access to healthcare services, including neurology devices
- Rising healthcare expenditure: The Brazilian government and private sector are investing more in healthcare, including the purchase of neurology devices, which is driving the growth of the market
- Technological advancements: Advances in technology are driving the development of new and improved neurology devices, which are being adopted in Brazil and contribute to the growth of the market
Market Restraints
There are several factors that may limit the growth of the neurology device market in Brazil, like:
- High cost of devices: The cost of neurology devices can be high, which may limit their accessibility for some patients and limit the growth of the market
- Limited reimbursement: Reimbursement for neurology devices by public and private insurance can be limited in Brazil, which may also limit their accessibility for some patients
- Lack of trained professionals: There may be a shortage of trained professionals in Brazil who are able to implant and use neurology devices, which may limit the growth of the market
- Regulatory hurdles: The regulatory environment for medical devices in Brazil can be complex and challenging, which may limit the introduction of new neurology devices and limit the growth of the market
- Economic instability: The Brazilian economy can be unstable at times, which may limit the growth of the healthcare sector, including the market for neurology devices
Competitive Landscape
Key Players
- Neuroservice (BRA)
- Neurosoft (BRA)
- NeuroRx (BRA)
- Fleury Medicina e Saúde (BRA)
- Hospital Albert Einstein (BRA)
- Hospital do Coração (BRA)
- Hospital Israelita Albert Einstein (BRA)
- Medtronic
- Boston Scientific
- Johnson & Johnson
- Neuronetics
Healthcare Policies and Regulatory Landscape
In Brazil, the regulatory body for medical devices, including neurological devices, is the National Health Surveillance Agency (ANVISA). ANVISA is responsible for evaluating and approving the safety and effectiveness of medical devices before they are marketed in Brazil. Additionally, Anvisa sets standards and regulations for the manufacture, import, distribution, and use of medical devices, ensuring that they meet the highest safety and quality standards for patients. The manufacturer and distributor of medical devices, including neurological devices, are subject to a variety of regulations set by the National Health Surveillance Agency in Brazil. All medical devices must be registered with ANVISA and a license must be obtained before they can be marketed in Brazil. The manufacturer must implement and maintain a quality management system that complies with international standards, such as ISO 13485. The device must be labeled with information such as the manufacturer's name and address, device name, and instructions for use. The manufacturer must implement a post-market surveillance program to monitor the performance and adverse events associated with their devices, and report these to ANVISA.
For some medical devices, such as those for neurological use, clinical trials may be required to demonstrate the safety and effectiveness of the device. The manufacturer and distributor must comply with import and export regulations and obtain the necessary permits and certificates from ANVISA. Medical device advertising and promotion must comply with regulations and must not make false or misleading claims.
Reimbursement Scenario
In Brazil, reimbursement for neuro devices by private and public insurance varies. Public health insurance (SUS) provides reimbursement for certain neurology devices for patients with specific medical conditions. However, the availability and type of neurology devices covered by SUS may be limited. On the other hand, private health insurance plans generally offer a wider range of coverage for neurology devices but the specifics of coverage will depend on the individual plan. Neurostimulation devices and Interventional Neurology devices are reimbursed by both private and public insurance in Brazil. However, the extent of coverage and specific devices covered may vary among insurance providers.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Neurology Device Market Segmentation
The Neurology Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Neurostimulation
- ?Spinal Cord Stimulation Devices
- Deep Brain Stimulation Devices
- Sacral Nerve Stimulation
- Vagus Nerve Stimulation
- Gastric Electric Stimulation
- Interventional Neurology
- Aneurysm Coiling & Embolization
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Cerebral Balloon Angioplasty & Stenting
- Carotid Artery Stents
- Filter Devices
- Balloon Occlusion Devices
- Neurothrombectomy
- Clot Retriever
- Suction Aspiration Devices
- Snares
- CSF Management
- CSF Shunts
- CSF Drainage
- Neurosurgery Devices
- Ultrasonic Aspirators
- Stereotactic Systems
- Neuroendoscopes
- Aneurysm Clips
By End User (Revenue, USD Billion):
- ??Hospitals and Clinics
- Specialty Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































