Brazil Lipid Disorder Therapeutics Market Analysis
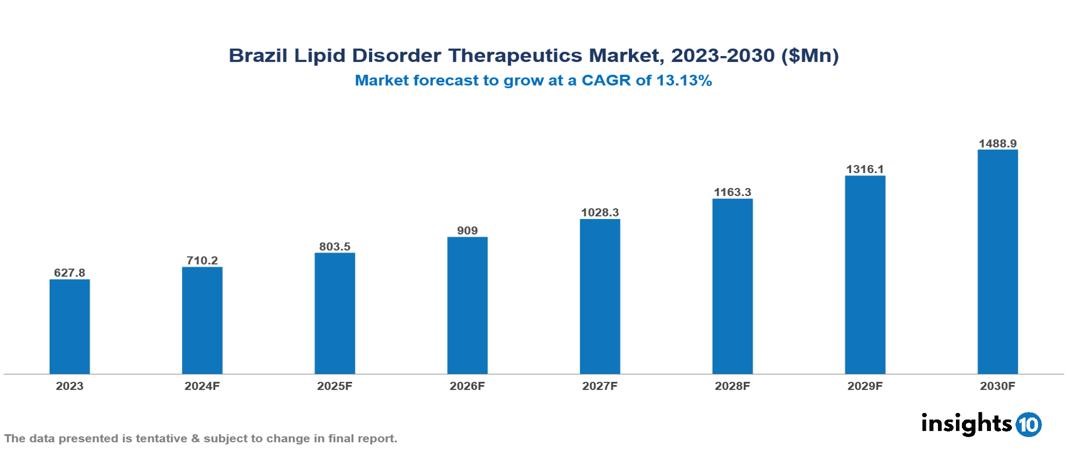
The Brazil Lipid Disorder Therapeutics Market was valued at $627.8 Mn in 2023 and is predicted to grow at a CAGR of 13.13% from 2023 to 2030, to $1,488.9 Mn by 2030. Brazil Lipid Disorder Therapeutics Market is growing due to the Rising Prevalence of Cardiovascular Diseases, the ageing population, and Advancements in Therapeutics and Technologies. The industry is primarily dominated by players such as Sanofi, Sun Pharmaceutical Industries Ltd. Pfizer, Inc., GlaxoSmithKline plc, Novartis AG, Merck & Co., Inc., Amgen Inc., Takeda Pharmaceutical Company Limited, AbbVie Inc., Viatris, AstraZeneca PLC, and Dr. Reddys Laboratories Ltd.
Buy Now

Brazil Lipid Disorder Therapeutics Market Executive Summary
Brazil's Lipid Disorder Therapeutics Market is at around $627.8 Mn in 2023 and is projected to reach $1,488.9 Mn in 2030, exhibiting a CAGR of 13.13% during the forecast period.
The several kinds of fats found in blood are called lipids, or lipoproteins. Lipid diseases are a broad category of metabolic ailments that affect blood lipid levels. The heightened blood levels of lipoproteins, triglycerides, and/or cholesterol that they share are associated with an increased risk of cardiovascular disease, if it occurs at all. Low-density lipoproteins (LDLs) can be lowered by following several easy steps, including cutting back on foods high in saturated fat, cholesterol, and calories; exercising; maintaining a healthy weight; and quitting smoking. Statins are the drugs that are most frequently prescribed to address lipids.
Prevalence of lipid alterations was 20.3%, reaching 42.9% in those who were obese. Demographic factors such as an aging population and urbanization contribute significantly to this increase. Healthcare expenditure is growing, with Brazil spending around 9.1% of its GDP on healthcare in 2022. This investment supports better access to treatments and diagnostic tools for lipid disorders. The market therefore is driven by significant factors like the Rising Prevalence of Cardiovascular Diseases, the ageing population, and Advancements in Therapeutics and Technologies. However, Preventative Healthcare, Side effects, and High cost of drugs restrict the growth and potential of the market.
In March 2023, the US Food and Drug Administration (FDA) authorized the extended use of Regeneron Pharmaceuticals, Inc.'s Evkeeza medication in children aged 5 to 11 years to treat an extremely rare condition that raises cholesterol levels.
Market Dynamics
Market Growth Drivers
Rising Prevalence of Cardiovascular Diseases: Dyslipidemia is a major risk factor for cardiovascular diseases (CVDs), which continue to be the leading cause of mortality. The need for cholesterol-lowering treatments is fueled by the rising prevalence of CVDs since controlling lipid levels is essential for preventing heart attacks and strokes. The need for efficient therapies for lipid disorders is heightened by the fact that about one-third of adults have excessive cholesterol, according to the Centers for Disease Control and Prevention (CDC).
Ageing Population: The elderly population is particularly vulnerable to lipid abnormalities and related cardiovascular problems. The prevalence of diseases like hypercholesterolemia and hypertriglyceridemia rises with the number of senior people, which raises the need for lipid disorder therapies to successfully treat these ailments.
Advancements in Therapeutics and Technologies: The development of new lipid-lowering drugs and improved diagnostic tools, such as advanced lipid panel testing kits and analyzers, have enhanced the capability to effectively manage and treat lipid disorders. Pharmaceutical companies' focus on research and development in this area is driving market expansion.
Market Restraints
Preventative Healthcare: Brazil healthcare emphasizes preventative measures to manage dyslipidemia. This includes dietary changes, exercise promotion, and weight management. While these approaches can be highly effective, behaviour modification can be challenging for some patients, potentially limiting the sole reliance on medication for managing lipid disorders.
Side effects: The side effects of cholesterol-lowering drugs are one of the main obstacles facing the lipid disease therapeutics market. Common medications like statins can cause side effects like elevated blood sugar, liver damage, and muscle soreness, which can discourage patients and restrict market growth. Because of these side effects, patients must be closely monitored and managed, which raises healthcare expenses and complicates treatment regimens.
High cost of drugs: Since lipid synthesis is complicated and requires high-quality raw materials, developing and producing lipid-based medications comes at a significant financial expense. Because customers frequently pay for these expenses, fewer people can afford these therapies. Generally, the market expansion might be restricted by the high cost, which can be a major obstacle, particularly for patients who lack sufficient insurance coverage.
Regulatory Landscape and Reimbursement Scenario
The Brazilian Health Regulatory Agency (ANVISA), which oversees healthcare goods and services from clinical trial authorization to post-marketing surveillance, is responsible for granting marketing authorization (MA) in Brazil. The 10-year MA by Anvisa certifies that medications are high-quality, safe, and effective. Companies must also apply for maximum pricing clearance from the Drug Market Regulatory Chamber (CMED) in order to be permitted to enter the Brazil market.
All citizens and visitors, including those without legal status, have free and full access to a range of services, including hospital, mental health, outpatient speciality, primary, and prescription drug coverage. There is no need for an application procedure. When it comes to medical treatments, there is no cost-sharing. Approximately 25% of Brazilians, primarily those from middle-class and upper-class backgrounds, possess private health insurance in order to avoid obstacles when seeking medical attention. Costs associated with purchasing health-related items and private health insurance are deductible from taxes.
Competitive Landscape
Key Players
Here are some of the major key players in the Brazil Lipid Disorder Therapeutics Market:
- Sanofi
- Sun Pharmaceutical Industries Ltd.
- Pfizer, Inc.
- GlaxoSmithKline plc
- Novartis AG
- Merck & Co., Inc.
- Amgen Inc.
- Takeda Pharmaceutical Company Limited
- AbbVie, Inc.
- Viatris
- AstraZeneca PLC
- Dr. Reddy’s Laboratories Ltd.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Lipid Disorder Therapeutics Market Segmentation
By Drug Type
- Atorvastatin
- Fluvastatin
- Simvastatin
- Pravastatin
- Others
By Indication
- Hypercholesterolemia
- Dysbetalipoproteinemia
- Familial Combined Hyperlipidemia
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.