Brazil Exosome Research Market Analysis
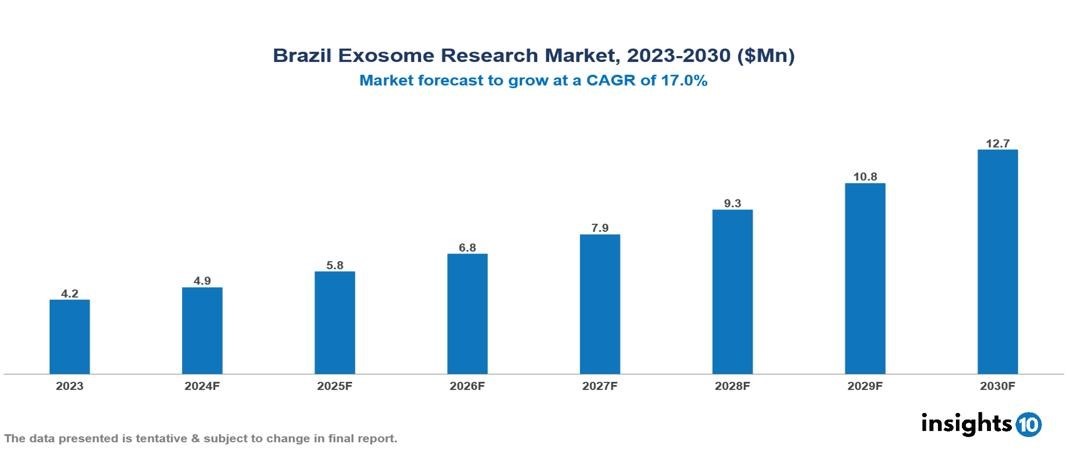
The Brazil Exosome Research Market was valued at $4.2 Mn in 2023 and is projected to grow at a CAGR of 17% from 2023 to 2023, to $12.7 Mn by 2030. The key drivers of this industry are increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, and increasing advanced applications of exosomes. The industry is primarily dominated by players such as Thermo Fisher Scientific, Bio-Techne Crop, QIAGEN, Beckam Coulter, Lonza among others.
Buy Now

Brazil Exosome Research Market Executive Summary
The Brazil Exosome Research Market is at around $4.2 Mn in 2023 and is projected to reach $12.7 Mn in 2030, exhibiting a CAGR of 17% during the forecast period 2023-2030.
Exosomes, tiny vesicles secreted by cells, are revolutionizing multiple fields due to their role in cell-to-cell communication and molecular transport. These nanoscale particles, packed with proteins, lipids, and genetic material, have captured the attention of researchers and industries alike for their diverse applications. In medicine, exosomes show promise as innovative drug delivery systems, potentially enhancing treatment precision while minimizing side effects. Their natural ability to traverse biological barriers makes them ideal candidates for targeted therapies in various diseases, including cancer and neurological disorders. Notably, exosomes derived from stem cells are being investigated for their regenerative and anti-inflammatory properties, with potential applications in treating organ damage and respiratory conditions like COVID-19.
The diagnostic sector is exploring exosomes as biomarkers for early disease detection and monitoring. This could lead to the development of non-invasive diagnostic tools, particularly valuable in fields such as oncology where liquid biopsies could revolutionize patient care. Cosmetic companies are also tapping into the potential of exosomes, especially those from stem cells, for skin rejuvenation products. These exosomes may deliver beneficial compounds to skin cells, promoting a more youthful appearance.
In the research arena, exosomes serve as crucial tools for understanding cellular interactions and disease mechanisms. They provide a window into the complex world of intercellular communication, potentially leading to groundbreaking discoveries in biology and medicine. The exosome market is poised for significant growth, driven by their versatility, biocompatibility, and ability to carry diverse therapeutic payloads without triggering immune responses. As isolation and engineering techniques improve, exosomes are likely to play an increasingly important role in personalized medicine, drug delivery innovations, and regenerative therapies, heralding a new era in biotechnology and healthcare.
The cancer mortality rate in Brazil is 37%, There were approximately 704,000 new cases of cancer expected for the triennium 2023-2025, except for non-melanoma skin cancer, 483,000 new cases will occur. The market therefore is driven by significant factors like increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, increasing advanced applications of exosomes.
Aethlon Medical, Inc., a medical therapy company focused on developing products for the diagnosis and treatment of cancer and life-threatening infectious diseases. Some of the major players operating in the Brazil Exosome Research Market are Hologic Inc., Luminex Corporation, Thermo Fisher Scientific, Agilent Technologies, Inc., Rooster Bio, Inc., Cell Guidance Systems LLC, QIAGEN, FUJIFILM Irvine Scientific, Cusabio Technology LLC, Nexosome-Oncology, Norgen Biotek Corp., Miltenyi Biotec, Aethlon Medical, Inc., and others.
Market Dynamics
Market Drivers
Increasing prevalence of chronic diseases: Brazil has a growing burden of chronic diseases like cancer, cardiovascular diseases, and neurodegenerative disorders, The cancer mortality rate in Brazil is 37%, There were approximately 704,000 new cases of cancer expected for the triennium 2023-2025, except for non-melanoma skin cancer, 483,000 new cases will occur, which are driving the demand for advanced diagnostics and therapeutics, including exosome-based solutions.
Growth in biomedical research: Brazil has a strong focus on biomedical research, with several universities and research institutions actively involved in exosome research, which can contribute to the development of exosome-based products and technologies.
Aging population: Brazil's population is aging, leading to a higher incidence of age-related diseases, which can drive the demand for exosome-based therapies that offer potential regenerative and therapeutic benefits.
Market Restraints
Regulatory challenges: The exosome market is relatively new, and there may be regulatory hurdles and challenges in obtaining approvals for exosome-based products in Brazil, which could hinder market growth.
Limited infrastructure and expertise: Brazil may have limited infrastructure and expertise in the field of exosome research and development, which could slow down the growth of the exosome market in the country.
High costs and limited reimbursement: Exosome-based therapies are often expensive, and the lack of adequate reimbursement policies or coverage by healthcare providers in Brazil could limit patient access and market adoption.
Regulatory Landscape and Reimbursement Scenario
ANVISA is an autarchy (semi-autonomous government agency) linked to the Brazilian Ministry of Health and part of the Brazilian National Health System (SUS). It serves as the coordinator of the Brazilian Health Regulatory System (SNVS) and has a presence throughout the national territory. ANVISA is responsible for the approval and supervision of food, cosmetics, tobacco, pharmaceuticals, health services, and medical devices, among others. It functions as the Brazilian counterpart to the U.S. Food and Drug Administration (FDA), regulating all health-related products. While some low-risk products may be exempt, it is mandatory to have a local importer or distributor for product liability purposes. Foreign companies are recommended to have technical staff and replacement parts available locally for customer support. The Brazilian healthcare market is price-driven, with domestically manufactured products having a distinct price advantage. Meeting quality and regulatory standards is important for products to be approved by ANVISA. Companies must meet all sanitary registration requirements set by ANVISA to sell products to the government. Foreign companies should consider cost-saving measures and highlight the benefits of new technologies in their marketing and promotional materials to compete in the Brazilian market.
Competitive Landscape
Key Players
Here are some of the major key players in the Brazil Exosomes Research Market:
- Thermo Fisher Scientific
- NanoSomix
- NX Pharmagen
- Malvern Instruments
- Miltenyi Biotec
- Codiak BioSciences Inc.
- Aethlon Medical, Inc.
- AGC Biologics
- Anjarium Biosciences AG
- Aruna Bio
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Exosomes Research Market Segmentation
By Products
- Instruments
- Software
- Reagents and Kits
By Applications
- Diagnostics
- Therapeutic
By Indication
- Cancer
- Cardiovascular Disease
- Neurogenerative Disease
- Infectious Disease
- Others
By Key End Users
- Hospitals
- Cancer Institutes
- Diagnostic Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.