Brazil Ear Infection Therapeutics Market Analysis
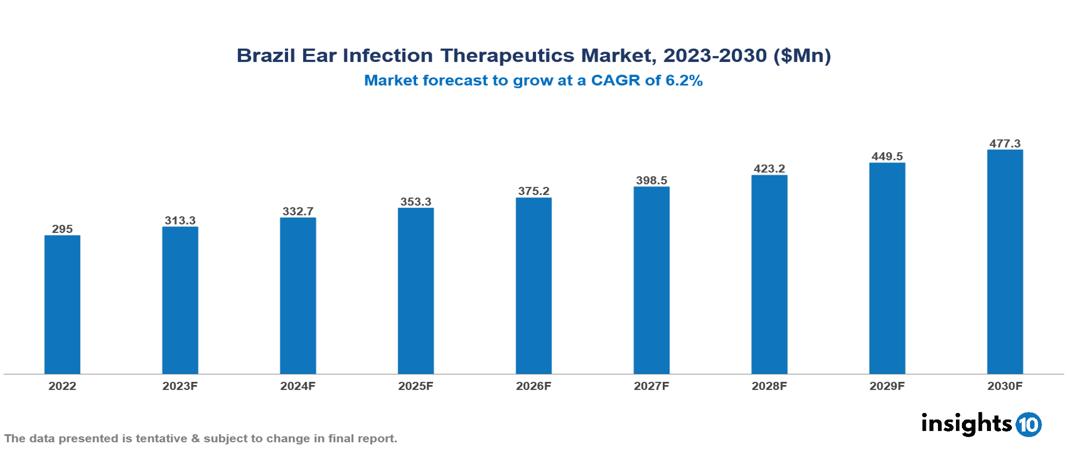
Brazil Ear Infection Therapeutics Market valued at $295 Mn in 2022, is projected to reach $477 Mn by 2030 with a 6.2% CAGR. The increasing prevalence of ear infections, especially in children, is a major factor contributing to the expansion of the market for therapeutics aimed at treating ear infections. This surge serves as a driving force for substantial research, development efforts, and the introduction of various treatments to tackle this prevalent medical issue. Prominent players in this industry include Pfizer, Novartis, Roche, Johnson & Johnson, Bayer, Merck & Co., AstraZeneca, Sanofi, GlaxoSmithKline, and Takeda Pharmaceutical Company.
Buy Now

Brazil Ear Infection Therapeutics Market Executive Summary
Brazil Ear Infection Therapeutics Market valued at $295 Mn in 2022, is projected to reach $477 Mn by 2030 with a 6.2% CAGR.
An ear infection, also known as otitis, refers to the inflammation or infection of the ear, typically affecting the middle ear. This condition can be caused by various factors, including bacteria or viruses. Common symptoms of an ear infection include ear pain, discomfort, hearing loss, and sometimes fluid drainage from the ear. Ear infections are prevalent, especially in children, but can occur in individuals of any age. Treatment often involves antibiotics for bacterial infections or supportive measures to manage symptoms. Seeking guidance from a healthcare professional is crucial for obtaining a precise diagnosis and the right treatment, as unaddressed ear infections may result in complications and the risk of hearing impairment.
Ear infections are a major public health concern in Brazil, that affects individuals across various age groups. The country's tropical climate and prevalent environmental conditions increase the susceptibility of the population to ear-related issues. Ear infections are particularly common in children, leading to a substantial health burden. In Southern Brazil, the prevalence of acute otitis media among children aged 0-5 years is notably high, with rates reaching 95.7 per 1000 persons overall. The incidence is even more pronounced in younger children, with a rate of 105.5 per 1000 persons for those aged 0-2 years and 63.6 per 1000 persons for those aged 3-5 years. Additionally, the prevalence of chronic otitis media in school-age children stands at 0.94% in Brazil. Addressing the impact of ear infections necessitates improvements in healthcare access, public awareness campaigns, and the development of effective therapeutics. The implementation of preventive measures remains integral in mitigating the occurrence and consequences of ear infections.
Researchers are looking into alternative therapies in response to the growing threat posed by bacteria that are resistant to antibiotics. This involves investigating viruses specifically targeting bacteria, known as bacteriophages, and immunomodulators, which strengthen the immune system's defense against infections. These innovative approaches offer potential solutions to the challenges posed by antibiotic resistance, highlighting the need to diversify strategies in infectious disease management.
Market Dynamics
Market Growth Drivers
Advancements in Treatment Options: Exploration of innovative drug delivery approaches such as intratympanic injections and the investigation of alternative treatments like bacteriophages to address antibiotic resistance present optimistic opportunities for the expansion of the market.
Increasing Prevalence of Ear Infections: The increasing prevalence of ear infections, particularly in the pediatric demographic, is a major catalyst propelling the expansion of the Brazil Ear Infection Therapeutics Market. This surge in ear infection cases underscores the need for advanced therapeutic solutions.
Growing Awareness and Early Diagnosis: Enhanced public awareness about ear infections and their prompt identification, facilitated by campaigns and educational programs, has the potential to result in timelier treatment and potentially increased demand for associated therapeutics.
Market Restraints
Antibiotic Resistance Concerns: The rising issue of antibiotic resistance poses a significant restraint on the ear infection therapeutics market, challenging the efficacy of traditional antibiotic treatments and necessitating the development of alternative solutions.
Stringent Regulatory Environment: The stringent approval process of new medications by the Brazilian Health Regulatory Agency (ANVISA) can result in delays in making potentially more effective treatments accessible compared to other nations.
Potential for Over-the-counter (OTC) Medications: The increasing accessibility and utilization of over-the-counter pain relievers and home remedies for minor ear infections could influence the demand for prescription medications to a certain degree.
Healthcare Policies and Regulatory Landscape
The National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA) in Brazil oversees healthcare policies and the control of medicinal products and also plays an important role in guaranteeing the quality, safety, and efficacy of drugs. Working in collaboration with ANVISA, the Chamber of Medicines evaluates the integration of new drugs into the public health system, while the Brazilian Health Regulatory Chamber (CMED) and the Brazilian Pharmaceutical Market Regulation Chamber contribute to the establishment of pricing regulations. The Ministry of Health collaborates with ANVISA to formulate healthcare strategies and policies, and the National Cancer Institute (INCA) is involved in developing cancer-related policies and guidelines. To ensure community involvement in health decisions, the National Health Council (CNS) plays a crucial role, while the National Commission for Ethics in Research (CONEP) oversees the ethical aspects of clinical trials.
Competitive Landscape
Key Players
- Pfizer
- Novartis
- Roche
- Johnson & Johnson
- Bayer
- Merck & Co.
- AstraZeneca
- Sanofi
- GlaxoSmithKline
- Takeda Pharmaceutical Company
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Ear Infection Therapeutics Market Segmentation
By Pathogen
- Bacteria
- Virus
- Fungus
By Treatment
- Surgery
- Medications
By Route of Administration
- Topical
- Oral
- Intravenous
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.