Brazil Dental Caries Detectors Market Analysis
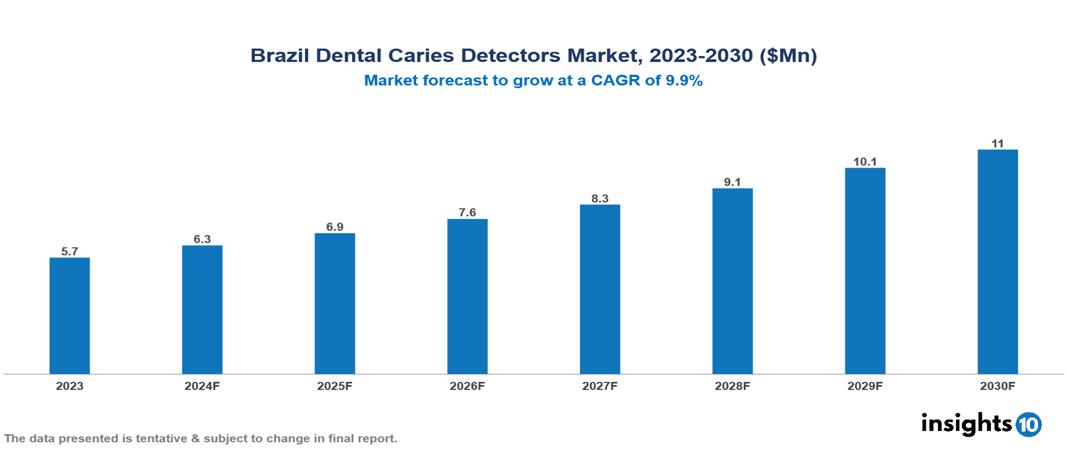
The Brazil Dental Caries Detectors Market was valued at $5.7 Mn in 2023 and is predicted to grow at a CAGR of 9.9% from 2023 to 2030, to $11 Mn by 2030. Brazil Dental Caries Detectors Market is growing due to the High prevalence of dental caries, Growing awareness, and Advanced imaging and diagnostics. The market is primarily dominated by players such as Sirona Dental Systems, Dentsply Sirona, KaVo Kerr, Ivoclar Vivadent, Planmeca Oy, Adec Technologies, 3M ESPE, Morita Corporation, Woodpecker Medical Instruments Co., Softdent.
Buy Now

Brazil Dental Caries Detectors Market Executive Summary
Brazil Dental Caries Detectors Market is at around $5.7 Mn in 2023 and is projected to reach $11 Mn in 2030, exhibiting a CAGR of 9.9% during the forecast period.
Dental caries, or tooth decay, is the most prevalent oral health condition. Cavities arise from the bacterial erosion of tooth enamel and dental health caused by a variety of factors, such as insufficient calcium or poor brushing technique. Using the caries detection method, holes in the proximities of teeth should be found as soon as possible. As a result, dental caries detectors are essential for identifying the location, size, and depth of carious interproximal lesions. Effective treatment plans can be implemented using less sophisticated technology by using dental X-rays, lasers, transillumination, revealing solutions, or other methods.
The average prevalence of dental caries in Brazil is high. High prevalence rates of dental caries among the population, exacerbated by dietary habits and oral hygiene practices, drive the demand for effective detection technologies. However, limited healthcare expenditures allocated to dental care and disparities in access across demographic groups hinder market growth. Socioeconomic factors also play a crucial role, with income disparities impacting affordability and adoption of advanced detection tools. These challenges underscore the complex landscape shaping the Brazilian dental caries detectors market. The market, therefore, is driven by significant factors like the High prevalence of dental caries, Growing awareness, and Advanced imaging and diagnostics. However, Rising Cost of treatment, Regulatory and Approval Challenges, Reimbursement Policies challenges restrict the growth and potential of the market.
Air Techniques Inc., an important producer, and leader of dental equipment, launched ScanX Classic View, a state-of-the-art digital radiography structure.
Market Dynamics
Market Growth Drivers
High Prevalence of dental caries: Brazil is among the countries with a higher frequency range in oral diseases, highlighting dental caries, which affect more than 90% of the Brazil population. Naturally, as this issue becomes more prevalent, there is a greater demand for efficient diagnostic tools like caries detectors, which help dentists effectively manage and cure caries.
Growing awareness: increasing awareness among the general public of the value of preventative care and oral health. The desire for routine dental examinations, which include caries detection, is being driven by this understanding. Early dental caries detection and treatment can reduce long-term healthcare costs and improve patient outcomes by preventing more serious tooth issues. Dental offices are being prompted by this tendency to use state-of-the-art caries detection equipment.
Advanced imaging and diagnostic: With the development of dental imaging and diagnostic technologies, more advanced caries detection equipment is now accessible. These tools detect cavities far more quickly than those that use radiographic imaging, fluorescence, and transillumination. The growing desire for more accurate and efficient diagnostic tools among dental practitioners is driving up the need for sophisticated caries detectors.
Market Restraints
Rising Cost of Treatment: Advanced dental caries detection devices, such as laser fluorescence and digital imaging tools, are often costly. For example, the average price of a laser fluorescence device can exceed $10,000, which can be prohibitive for smaller dental practices and clinics. This high cost limits their ability to invest in these technologies, resulting in many practitioners continuing to use traditional methods. This reluctance to adopt innovative detection solutions affects the overall market growth. The initial investment and ongoing maintenance costs can be a significant financial burden, particularly for practices operating with tight budgets. As a result, the widespread adoption of advanced caries detection technology remains sluggish, hindering potential market expansion.
Regulatory and Approval Challenges: The approval process for new dental devices by regulatory bodies can be lengthy and stringent. This regulatory scrutiny ensures safety and efficacy but can delay the introduction of innovative caries detection tools to the market. The time and resources required for compliance with regulatory standards can be a barrier for manufacturers, potentially slowing down the availability of new technologies and impeding market growth.
Reimbursement Policies challenges: Insurance companies often provide limited or no reimbursement for dental procedures involving advanced caries detection technologies. For instance, only about 30% of insurance plans in cover advanced diagnostic tools for dental care. This lack of coverage can discourage both dentists and patients from opting for these modern diagnostic tools. The financial burden of out-of-pocket expenses can lead to a preference for traditional, less expensive methods, thereby restricting the widespread adoption of advanced detection devices in the market. Consequently, the financial constraints imposed by limited insurance coverage hinder the growth and integration of these advanced technologies in dental practices
Regulatory Landscape and Reimbursement Scenario
In Brazil, the regulatory landscape for dental caries detectors is governed by strict standards set by the National Health Surveillance Agency (ANVISA). ANVISA mandates that all dental devices, including caries detectors, must undergo rigorous testing and registration processes to ensure safety, efficacy, and quality before they can be marketed and used in the country. These regulations aim to protect public health by ensuring that dental professionals have access to reliable and effective tools for diagnosing and treating dental caries. Compliance with ANVISA's regulations is essential for manufacturers and importers seeking to enter and maintain a presence in Brazil's dental care market.
In Brazil's dental caries detectors market, reimbursement mechanisms play a critical role in influencing adoption among dental professionals. The country's public healthcare system, SUS (Sistema Único de Saúde), provides coverage for basic dental procedures, but coverage for advanced diagnostic technologies like digital imaging or fluorescence-based systems may vary. Private insurance plans often offer more comprehensive coverage, affecting the adoption of newer detection methods in private practices. Proper adherence to specific billing codes and regulations is essential for reimbursement. Challenges include ensuring equitable access across socio-economic groups and updating policies to reflect advancements in dental technology, which impact widespread adoption in the Brazilian market.
Competitive Landscape
Key Players
Here are some of the major key players in the Brazil Dental Caries Detectors Market:
- Sirona Dental Systems
- Dentsply Sirona
- KaVo Kerr
- Ivoclar Vivadent
- Planmeca Oy
- Adec Technologies
- 3M ESPE
- Morita Corporation
- Woodpecker Medical Instruments Co.
- Softdent
- Elmeco
- Anthogyr
- Bien Air Dental
- FONA Dental
- Genoray International Co.
- I.N. Dental
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Dental Caries Detectors Market Segmentation
Based on Type
- Laser Fluorescent caries detector
- Fiber Optic
- Trans-Illumination Caries Detector
- Others
Based on the Distribution Channel
- Online Platforms
- Offline Platforms
Based on End-user
- Hospitals
- Dental clinics
- Ambulatory Surgical Centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































