Brazil Congestive Heart Failure Therapeutics Market Analysis
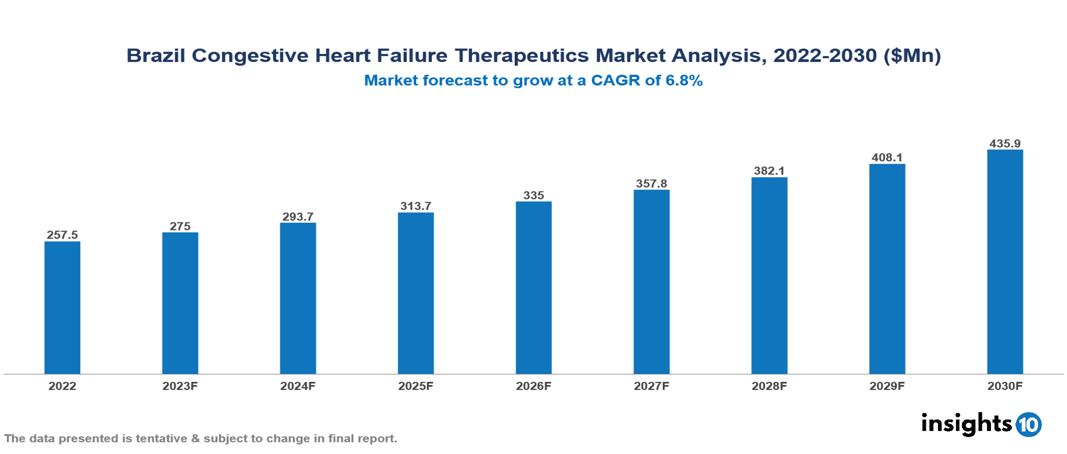
The Brazil Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $258 Mn in 2022 to $436 Mn by 2030, with a CAGR of 6.8% during the forecast period of 2022-2030. An expanding patient base due to rising cardiac disease prevalence, increased healthcare access around the country, and a thriving R&D environment are the key factors driving the market growth in Brazil. The Brazil Congestive Heart Failure Therapeutics Market encompasses various key players across different therapeutic segments, including Johnson & Johnson, Merck, Novartis, Pfizer, Sanofi, Hypera Pharma, Ache Laboratories, EMS Pharma, Neo Quimica, Teuto Brasileiro, etc, among various others.
Buy Now

Brazil Congestive Heart Failure Therapeutics Market Analysis Executive Summary
The Brazil Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $258 Mn in 2022 to $436 Mn by 2030, with a CAGR of 6.8% during the forecast period of 2022-2030.
Congestive Heart Failure (CHF) is a condition characterized by the heart's inability to efficiently pump blood, resulting in insufficient circulation. Key causes include coronary artery disease, hypertension, and prior heart attacks, all contributing to a weakened heart muscle. The two primary types are systolic heart failure, impairing blood pumping, and diastolic heart failure, hindering the heart's ability to relax and fill with blood. Treatment strategies aim to alleviate symptoms, enhance heart function, and address underlying issues. Commonly prescribed medications include ACE inhibitors, beta-blockers, and diuretics. Lifestyle adjustments, like a low-sodium diet, regular exercise, and weight management, play a crucial role. In severe cases, interventions such as implantable devices or heart transplants may be considered. Emerging approaches like gene therapy, stem cell therapy, and precision medicine offer promising prospects for addressing CHF's root causes and improving patient outcomes. Furthermore, innovative drug designs targeting specific heart failure mechanisms are advancing into clinical trials, providing hope for more effective and personalized treatment options.
Heart failure (HF) affects an estimated 4.3 Mn people in Brazil, accounting for around 2% of the adult population. The prevalence of heart failure is predicted to grow due to the aging population and the increased incidence of cardiovascular disease.
An expanding patient base due to rising cardiac disease prevalence, increased healthcare access around the country, and a thriving R&D environment are the key factors driving the market growth in Brazil.
Novartis is a market leader in the Brazil market, with great brand awareness and a diversified product range that includes the best-selling CHF drug Entresto. Bayer has a substantial market share with its established CHF medicine Coreg. AstraZeneca is becoming more popular among specific patient categories in the CHF market, owing to its well-known drug, Brilinta.
Market Dynamics
Market Growth Drivers
Growing Market Opportunities: The expanding prevalence of CHF in Brazil, driven by factors such as an aging population, unhealthy lifestyles, and increased obesity rates, contributes to an augmented patient base. This escalating patient population enhances the potential market size, making entry into the CHF medication market more appealing and financially rewarding.
Increased Healthcare Accessibility: Government-driven initiatives and the expansion of private insurance plans are fostering greater access to healthcare services. This enhanced accessibility empowers a larger segment of the population to afford CHF treatment. The broadening reach of patients provides a compelling incentive for pharmaceutical companies to participate in and explore opportunities within the market.
Thriving Research and Development Environment: Brazil is experiencing a notable surge in investment in research and development (R&D) as well as clinical trials, creating an attractive landscape for companies interested in advancing innovative CHF therapies. This increased commitment to R&D signifies a proactive approach towards understanding and addressing the complexities of CHF. Companies are drawn to this flourishing environment with the prospect of not only developing but also commercializing cutting-edge therapies.
Market Restraints
Highly Competitive Landscape: The market is already crowded with established global and domestic players, making it challenging for new entrants to gain market share. Companies need to differentiate themselves through innovative products, competitive pricing strategies, or targeted marketing efforts to stand out in the competition.
Limited Reimbursement: Public and private insurance coverage for CHF medications can be limited, creating challenges for patients to access expensive therapies. Companies need to focus on demonstrating the cost-effectiveness of their products and securing favorable reimbursement policies to ensure patient access and market success.
Logistical and Distribution Challenges: Brazil's vast and diverse geography, coupled with complex logistics infrastructure, can make it difficult and expensive to distribute medications nationwide. Companies need to establish efficient distribution networks and partnerships to ensure their products reach patients across the country, especially in remote or underserved areas.
Healthcare Policies and Regulatory Landscape
Brazil's National Health Surveillance Agency, ANVISA, works under the Ministry of Health and is responsible for managing and regulating different sectors of the country's health and sanitation sector. The primary goal of ANVISA is to protect and improve public health by ensuring the safety and efficacy of healthcare products, services, and systems. ANVISA is in charge of licensing and monitoring a wide range of products, including pharmaceuticals, medical devices, food and drinks, and cosmetics, before they enter the Brazilian market, guaranteeing compliance with both national legislation and international standards. The organization works hard to ensure that healthcare products available to Brazilians fulfill high quality, safety, and efficacy criteria through rigorous inspections and regulatory measures. In addition to product regulation, ANVISA actively participates in epidemiological surveillance, health trend monitoring, and public health emergency response. The organization works with both national and international partners to stay up to date on healthcare developments and contribute to global health efforts.
Competitive Landscape
Key Players:
- Johnson & Johnson
- Merck
- Novartis
- Pfizer
- Sanofi
- Hypera Pharma
- Ache Laboratories
- EMS Pharma
- Neo Quimica
- Teuto Brasileiro
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.