Brazil Chronic Pain Therapeutics Market Analysis
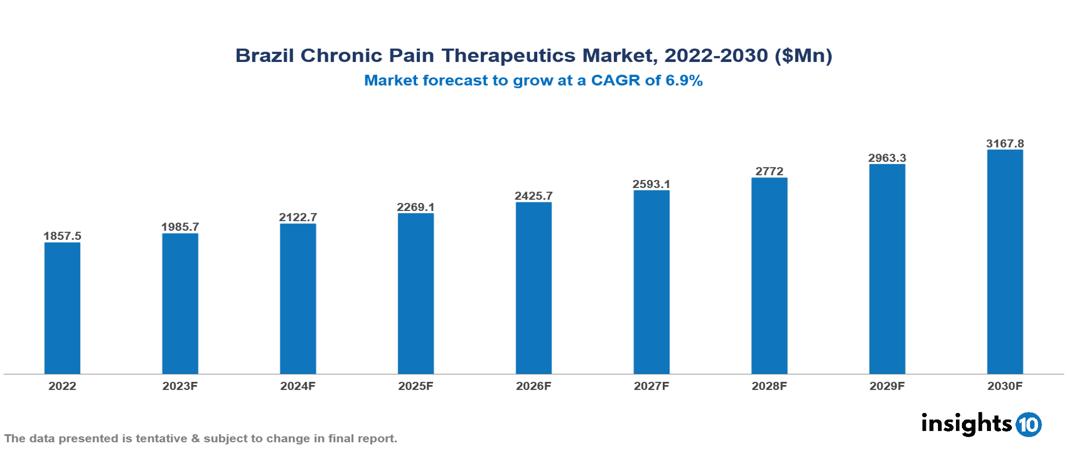
The Brazil Chronic Pain Therapeutics Market is anticipated to experience a growth from $1.858 Bn in 2022 to $3.168 Bn by 2030, with a CAGR of 6.9% during the forecast period of 2022-2030. The drivers of the Brazil Chronic Pain Therapeutics Market include the increasing incidence among the population owing to changing urban lifestyles, continuous advancements in pain management treatments, and crucial regulatory support and healthcare policies implemented by the Brazilian government. The Brazil Chronic Pain Therapeutics Market encompasses various players across different segments, including AbbVie, Pfizer, Novartis, Merck, GSK, Sanofi, Roche, Hypera Pharma, EMS, Biolab Pharma, etc., among various others.
Buy Now

Brazil Chronic Pain Therapeutics Market Analysis Executive Summary
The Brazil Chronic Pain Therapeutics Market is anticipated to experience a growth from $1.858 Bn in 2022 to $3.168 Bn by 2030, with a CAGR of 6.9% during the forecast period of 2022-2030.
Chronic pain refers to persistent discomfort that lasts for long periods, often surpassing the anticipated healing duration of an injury or illness, spanning weeks, months, or even years. It can stem from conditions like arthritis, fibromyalgia, neuropathy, migraines, and back injuries. Pain types include nociceptive (tissue damage), neuropathic (nerve damage), and idiopathic (unknown cause). Addressing chronic pain typically involves a comprehensive approach tailored to the individual's requirements. This may encompass managing medication with analgesics, antidepressants, or anticonvulsants to alleviate pain and enhance mood. Physical therapy and exercise routines play a crucial role in improving mobility and strengthening muscles, thereby reducing strain on affected areas. Psychological interventions, such as cognitive-behavioral therapy (CBT) and mindfulness-based techniques, aim to address the emotional and mental aspects of pain, aiding individuals in better coping. Additionally, interventional procedures like nerve blocks, spinal cord stimulation, or injections may be considered to provide targeted relief for pain.
In Brazil, the general adult population has a significant incidence of chronic pain (35.7%), whereas the senior group has a higher prevalence (47.3%). Also, female sex, aging, low educational attainment, high levels of professional engagement, excessive alcohol use, smoking, central obesity, mood disorders, and sedentarism are all risk factors and are more linked to chronic pain. There was a greater incidence of chronic pain in Brazil's southern and southeast areas.
The drivers of the Brazil Chronic Pain Therapeutics Market include the increasing incidence among the population owing to changing urban lifestyles, continuous advancements in pain management treatments, and crucial regulatory support and healthcare policies implemented by the Brazilian government.
The biggest market shares in Brazil are held by multinational pharma companies like AbbVie, Pfizer, Novartis, and MSD because of their well-established global footprint. Brazilian firms that serve the local population and needs, such as EMS and Hypera Pharma, have a larger market share in particular therapeutic categories.
Market Dynamics
Market Growth Drivers
Changing lifestyles: As urban areas expand and individuals increasingly adopt sedentary behaviors, maintain poor postures, and contend with heightened stress levels, the incidence of chronic pain issues is on the rise. Sedentary lifestyles, often associated with prolonged hours spent sitting or engaging in limited physical activity, contribute to musculoskeletal problems and discomfort. Poor posture, exacerbated by the use of technology and long hours at desks, further compounds these issues. The escalating levels of stress, characteristic of modern urban living, also play a role in the development and exacerbation of chronic pain conditions.
Advancements in treatment: The field of pain management is witnessing continuous advancements due to robust research and development initiatives. Ongoing efforts are dedicated to exploring and introducing novel treatment modalities that offer increased efficacy and improved patient outcomes. These innovations, ranging from pharmaceutical breakthroughs to technological solutions, contribute substantially to the growth and evolution of the chronic pain therapeutics market.
Government support: Regulatory support and healthcare policies implemented by the Brazilian government play a pivotal role in influencing the accessibility and affordability of pain management solutions. Policies geared towards enhancing healthcare access and affordability not only address the immediate needs of individuals suffering from chronic pain but also contribute to the overall growth and sustainability of the chronic pain therapeutics market in Brazil.
Market Constraints
Lack of public awareness: The lack of understanding of chronic pain has become a big problem that leads to societal stigma and ignorance of the treatment options available hence deterring people from seeking prompt help. This unawareness not only delays the diagnosis of chronic pain conditions but also prevents the implementation of appropriate management strategies.
Economic problems: Moreover, limited insurance coverage contributes to high out-of-pocket expenses for chronic pain patients. Many pain medications are neither fully nor partly covered by private or public health insurance companies. As such, this financial pressure acts as a major obstacle in accessing necessary treatments that make it impossible for people with these ailments to manage them effectively.
Inadequate access to healthcare: In rural areas and among low-income groups, disparities in healthcare persist thereby limiting chances of specialized care provision and advanced therapies’ availability. Consequently, numerous individuals do not receive adequate attention or suffer preventable mistreatment leading to continued agony related to acute pain. For example, there is a scarcity of specialist doctors dealing with pain in Brazil with only about 2,000 attending to over 210 Mn Brazilians.
Healthcare Policies and Regulatory Landscape
The National Health Surveillance Agency or ANVISA (Agência Nacional de Vigilância Sanitária) is the Brazilian regulatory agency that is responsible for the approval and supervision of food, cosmetics, tobacco, pharmaceuticals, health services, and medical devices, among others. The agency is connected to the Ministry of Health, which manages ANVISA through a management contract signed periodically. Its primary mandate is to protect and promote public health by ensuring the safety and efficacy of health products, services, and processes. The agency plays a critical role in the registration and evaluation of these products before they enter the Brazilian market, ensuring compliance with national regulations and international standards. Through rigorous inspections and regulatory measures, it aims to guarantee the quality, safety, and effectiveness of healthcare products available to the Brazilian population. In addition to product regulation, it is actively involved in epidemiological surveillance, monitoring health trends, and responding to public health emergencies. The agency collaborates with national and international partners to stay abreast of advancements in healthcare and to contribute to global health initiatives.
Competitive Landscape
Key Players:
- AbbVie
- Pfizer
- Novartis
- Merck
- GSK
- Sanofi
- Roche
- Hypera Pharma
- EMS
- Biolab Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Chronic Pain Therapeutics Market Segmentation
By Indication
- Neuropathic Pain
- Back Pain
- Headaches
- Arthritis Pain
- Muscular Pain
- Idiopathic Pain
- Others
By Drug Class
- Analgesics
- Opioids
- NSAIDs
- Anaesthetics
- Others
By Route of Administration
- Oral
- Topical
- Parenteral
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.