Brazil Carboprost Tromethamine Market Analysis
Brazil Carboprost Tromethamine Market is projected to grow from $xx Mn in 2022 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2022 - 2030. The rising prevalence of postpartum haemorrhage, increased focus on maternal healthcare, developments in healthcare infrastructure, technological innovations in drug delivery, increasing maternal age, and supportive government initiatives are driving the carboprost tromethamine market. Pfizer Inc., Ferring Pharmaceuticals, JHP Pharmaceuticals, Hikma Pharmaceuticals, and Hemofarm A.D. are some of the leading global key competitors in the carboprost tromethamine market.
Buy Now

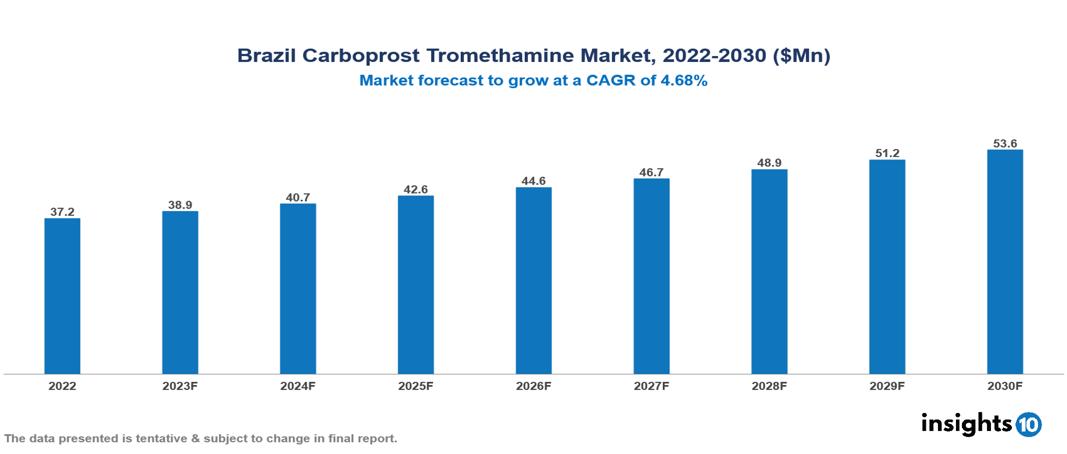
Brazil Carboprost Tromethamine Market Analysis Summary
Brazil Carboprost Tromethamine Market is valued at around $37.2 Mn in 2022 and is projected to reach $53.6 Mn by 2030, exhibiting a CAGR of 4.68% during the forecast period 2023-2030.
Carbopost is a synthesized oxytocic prostaglandin. Hemabate, also known as carboprost tromethamine, is a kind of prostaglandin used to treat postpartum hemorrhage and excessive bleeding after childbirth in women. Carboprost Tromethamine is usually delivered as an injection and only under medical supervision. It is a prescription-only drug that should only be used under the supervision of a healthcare practitioner. The rising prevalence of postpartum hemorrhage, increased focus on maternal healthcare, developments in healthcare infrastructure, technological innovations in drug delivery, increasing maternal age, and supportive government initiatives are driving the carboprost tromethamine market. Pfizer Inc., Ferring Pharmaceuticals, JHP Pharmaceuticals, Hikma Pharmaceuticals, and Hemofarm A.D. are some of the leading global key competitors in the carboprost tromethamine market.
Market Dynamics
Market Growth Drivers
- Increasing maternal healthcare: Maternal healthcare is becoming more important, and the necessity to handle difficulties during childbirth has increased demand for medications like Carboprost Tromethamine. Governments, healthcare institutions, and non-governmental organizations (NGOs) have been focusing on improving maternal health outcomes, which has led to an increase in the use of drugs like Carboprost Tromethamine to treat postpartum bleeding
- Population growth and birth rates: The world population is growing, which contributes to greater birth rates in many places. As the frequency of childbirth increases, so will the demand for drugs like carboprost tromethamine to treat postpartum bleeding
- Medical technology and procedure improvements: Technological advancements in the field of obstetrics and gynecology have resulted in enhanced diagnostic techniques and treatment choices. These developments have improved the efficacy and safety of therapies, including the use of medicines such as carboprost tromethamine
- Regulatory assistance and guidelines: Government rules and guidelines aimed at improving mother health and lowering maternal mortality have contributed to the growth of the market for drugs such as Carboprost Tromethamine. These laws encourage the use of approved drugs and methods, resulting in better postpartum hemorrhage care
- Increasing awareness and education: As healthcare professionals and patients become more aware of the need to control postpartum hemorrhage, the demand for drugs like Carboprost Tromethamine has increased. Healthcare practitioners are becoming more aware of treatment choices, which is contributing to an increase in the prescription and use of such pharmaceuticals
Competitive Landscape
Key Players
- Pfizer Inc.
- Ferring Pharmaceuticals
- JHP Pharmaceuticals
- Hikma Pharmaceuticals
- Hemofarm A.D.
Development in Carboprost Tromethamine Market
- Jan. 2023- Long Grove Pharmaceuticals launched a single-pack presentation of its second generic to market, carboprost tromethamine injection, USP
- Feb. 2023- Caplin Point Laboratories’ US subsidiary, Caplin Steriles Ltd., received final approval from the United States Food and Drug Administration (USFDA) for carboprost tromethamine injection, which is used for the treatment of postpartum hemorrhage due to uterine atony
- May 2023, Aurobindo Pharma subsidiary Eugia Pharma Specialties has received the final approval from the U.S. Food and Drug Administration (USFDA) to manufacture and market Carboprost Tromethamine Injection USP 250 mcg/mL, single-dose vials
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Carboprost Tromethamine Market Segmentations
By Application
- Postpartum Hemorrhage
- Abortion Induction
By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
By End-User
- Hospitals
- Clinics and Maternity Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































