Brazil Breast Pump Market Report
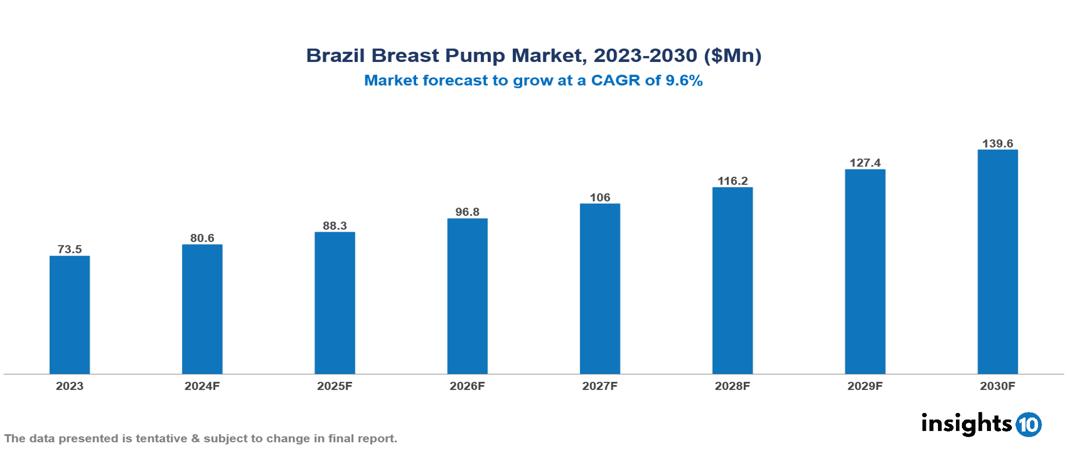
The Brazil Breast Pump Market was valued at $73.5 Mn in 2023 and is predicted to grow at a CAGR of 9.6% from 2023 to 2030, to $139.6 Mn by 2030. The key drivers of the market include improving healthcare infrastructure, growing consumer awareness, and rising disposable incomes. The prominent players in the Brazil Breast Pump Market are Ardo, CA-MI, Lansinoh, Microlife, Nubeca, and Nuby, among others.
Buy Now

Brazil Breast Pump Market Executive Summary
The Brazil Breast Pump Market is at around $73.5 Mn in 2023 and is projected to reach $139.6 Mn in 2030, exhibiting a CAGR of 9.6% during the forecast period.
A breast pump is a mechanical device that lactating women use to extract milk from their breasts. They are available in two forms; they can be manual devices powered by hand or foot movements or automatic devices powered by electricity. Breast pumps are available in a variety of styles to meet the demands of moms. Hand-operated manual pumps are lightweight and silent, making them ideal for sporadic usage. Electric pumps are often used on a regular basis because of their higher efficiency and ability to run on mains or batteries. There are several uses for breast pumps. Many parents use them to continue breastfeeding after they return to work. They express their milk at work, which is later bottle-fed to their child by a caregiver. A breast pump may be also used to address a range of challenges parents may encounter during breastfeeding, including difficulties latching, separation from an infant in intensive care, feeding an infant who cannot extract sufficient milk itself from the breast, to avoid passing medication through breast milk to the baby, or to relieve engorgement, which is a painful condition whereby the breasts are overfull.
The Brazil Breast Pump Market is driven by significant factors such as the improving healthcare infrastructure, growing consumer awareness, and rising disposable incomes. However, high cost, lack of insurance coverage, and stigma and embarrassment restrict the growth and potential of the market.
The major players in the Brazil Breast Pump Market are Ardo, CA-MI, Lansinoh, Microlife, Nubeca, and Nuby, among others.
Market Dynamics
Market Growth Drivers
Improving healthcare infrastructure: With the enhancement of the healthcare system, maternal and infant health has gained significant attention, leading to a heightened demand for breast pumps. Improved healthcare facilities now provide superior lactation support, education, and awareness about the advantages of breastfeeding, prompting more mothers to choose breast pumps. Furthermore, the growth of healthcare infrastructure in developing areas ensures that breast pumps are more accessible and affordable, driving market expansion and enabling more mothers to conveniently breastfeed, even in difficult situations.
Growing consumer awareness: The rising awareness among consumers is a crucial factor propelling the growth of the Breast Pump Market. With more accessible information about breastfeeding benefits and breast pump convenience from healthcare providers, online platforms, and social media, more mothers are being encouraged to use breast pumps. Advocacy from health organizations and government programs promoting breastfeeding for infant health further raises this awareness. Additionally, the modern need for parenting flexibility and workplace support for breastfeeding mothers have increased breast pump adoption rates, thereby driving market expansion.
Rising disposable incomes: The Breast Pump Market is significantly propelled by rising disposable incomes, which enhance the capacity of households to invest in quality healthcare products. With more financial flexibility, families are increasingly able to purchase advanced breast pump models that provide superior convenience, efficiency, and comfort. This economic uptrend not only allows for greater affordability but also fosters a heightened awareness and prioritization of health and wellness. As a result, the demand for breastfeeding support products grows, motivating manufacturers to innovate and expand their product lines, thereby driving further market expansion.
Market Restraints
High Cost: The high cost of advanced breast pumps can act as a significant market growth restraint. Despite the benefits of features such as hands-free operation, advanced technology, and higher efficiency, the high prices prevent usage from budget-conscious consumers. This is especially relevant in rural regions where healthcare reimbursements for breastfeeding equipment are limited. As a result, many potential buyers are lost, constraining the market growth.
Lack of Insurance Coverage: In Brazil, where many healthcare expenses are out-of-pocket, the absence of insurance support for breast pumps limits their accessibility and affordability for a significant portion of the population. This financial barrier often deters prospective buyers from investing in these essential breastfeeding tools, particularly among lower-income families. Without broader insurance coverage, the market for breast pumps in Brazil struggles to reach its full potential.
Stigma and Embarrassment: The stigma and embarrassment associated with using breast pumps can act as a restraint on the breast pump market’s growth. Social and cultural perceptions about breastfeeding and breast pump use can deter some women from utilizing these devices, particularly in public or workplace settings. The use of breast pumps causes discomfort with the visibility of the pump, concerns about judgment, or lack of support from employers and society. These societal attitudes can hinder broader adoption and acceptance, potentially limiting market growth.
Regulatory Landscape and Reimbursement Scenario
The Agência Nacional de Vigilância Sanitária (ANVISA), often known as the National Health Surveillance Agency, is the Brazilian regulatory body in charge of approving and monitoring a variety of products, including food, cosmetics, tobacco, medications, health services, and medical devices. ANVISA was created in 1999 and is linked to the Ministry of Health. ANVISA aims to safeguard and advance public health by conducting health surveillance over goods and services, including procedures, components, and technology that may present health concerns.
Pharmaceutical laboratories and other businesses that are a part of the pharmaceutical production cycle must register their drugs and obtain licenses from ANVISA. The agency is also in charge of creating rules that apply to clinical trials in the areas of subject safety and drug Chemistry, Manufacturing, and Control (CMC). Additionally, ANVISA collaborates with the Chamber of Drug Market Regulation (CMED) and other ministry members to control pharmaceutical prices. Human clinical trials conducted ethically are overseen by an Ethics Committee (EC) affiliated with the Health Ministry. The agency inspects manufacturers, monitors drug quality, conducts post-marketing surveillance, performs pharmacovigilance activities, and controls drug marketing and promotion in collaboration with states and local governments. Furthermore, ANVISA, in collaboration with the National Industrial Property Institute (INPI), assesses patent requests pertaining to pharmaceutical items and procedures. After becoming an ICH member in November 2016, Brazil sought to facilitate regulatory approval by implementing the Common Technical Document (CTD) for the registration of medicines.
Brazil’s dual-structure healthcare reimbursement system serves both the public and private sectors. All Brazilian citizens and permanent residents are eligible for free at-the-point healthcare coverage through the Unified Health System (SUS). The government provides direct money to SUS facilities and patients do not have their own reimbursement procedure. However, due to financial limitations, SUS may have problems including lengthy wait periods and restricted access to cutting-edge therapies. A significant portion of the population is covered by private health insurance, which provides a greater choice of treatments and faster wait times than SUS. Private insurance firms pay medical providers according to prearranged contracts or schedules of fees. Methods of reimbursement may differ but usually include: Fee-for-Service (FFS) which provides payment according to the quantity of services rendered; Diagnosis Groups (DRGs) assign a predetermined cost for treating a certain illness; and in Managed Care Networks, the providers under contract offer discounted services.
Competitive Landscape
Key Players
Here are some of the major key players in the Brazil Breast Pump Market:
- Ardo
- CA-MI
- Lansinoh
- Microlife
- Nubeca
- Nuby
- Elvie
- Bistos
- Ameda
- Avent
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Breast Pump Market Segmentation
By Product Type
- Manual pumps
- Battery-powered pumps
- Electric pumps
By Pump System
- Open System
- Closed System
By Pumping Type
- Single
- Double
By Distribution Channel
- Hospital Pharmacies
- Retail Stores
- Online Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































