Brazil Anemia Therapeutics Market Analysis
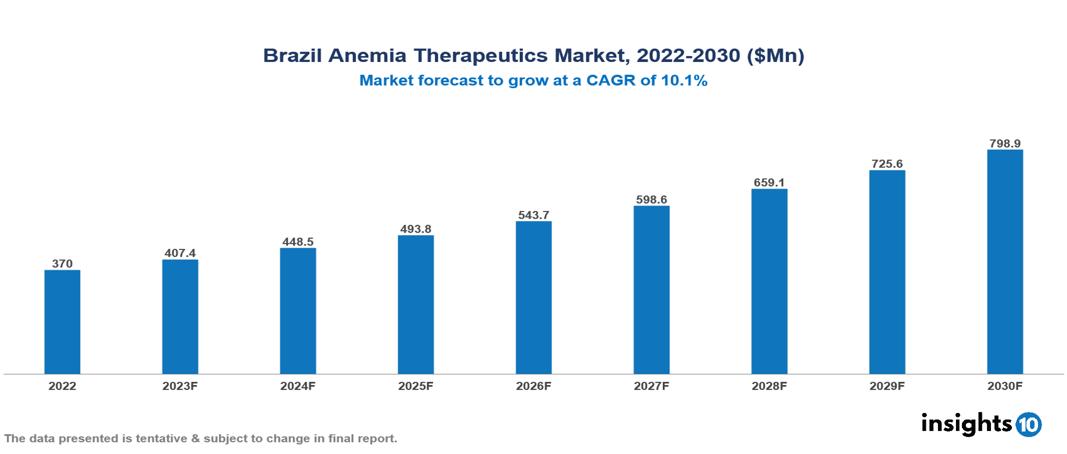
The Brazil Anemia Therapeutics Market is anticipated to experience growth from $370 Mn in 2022 to $799 Mn by 2030, with a CAGR of 10.10% during the forecast period of 2022–2030. Factors such as the demography and disease burden in Brazil, increasing adaptation of healthcare innovations and advancements, and lenient and fast regulatory policies, drive market growth. The Brazil Anemia Therapeutics Market encompasses various players across different segments, including Amgen, Roche, Novartis, Eli Lilly, Sanofi, Cristalia Pharma, Ache Laboratories, EMS Pharma, Eurofarma, BioLab Pharma, etc, among various others
Buy Now

Brazil Anemia Therapeutics Market Analysis Executive Summary
The Brazil Anemia Therapeutics Market is anticipated to experience growth from $370 Mn in 2022 to $799 Mn by 2030, with a CAGR of 10.10% during the forecast period of 2022–2030.
Anemia, an ailment described as lacking red blood cells or haemoglobin in the circulation system, brings about a diminished limit of blood to convey oxygen to substantial tissues. Hemoglobin, a protein tracked down in red blood cells, is responsible for oxygen restriction and transport. Anemia can be caused by a number of things, including iron deficiency, chronic illnesses, genetic factors, and some medical treatments. It can affect people of all ages because of food inconsistencies or underlying health issues. Treatment choices shift in view of the seriousness and fundamental reason. Tending to wholesome deficiencies, like iron, vitamin B12, or folic acid, may include supplementation or dietary changes. Iron enhancements are usually endorsed for lack of iron paleness (IDA). Ongoing illnesses require treating the underlying driver, while serious cases could require blood bonding. New erythropoiesis-stimulating agents (ESAs) and cutting-edge iron replacement therapies for patients who don't respond well to conventional treatments are the focus of ongoing research.
Anemia affects around 9.9% of Brazilian adults and the elderly (ages 18 and above). Anemia is more common in women than in men, with 12.3% of women and 7.2% of males suffering from it. Anemia is more prevalent in Brazil's north and northeastern areas than in the south and southeast. Iron deficiency is the leading cause of anemia in Brazil, followed by chronic illnesses such as renal disease and cancer.Factors such as the demography and disease burden in Brazil, the increasing adaptation of healthcare innovations and advancements, and lenient and fast regulatory policies, drive market growth.
Cristalia Pharma leads the market in terms of overall anemia medicine sales, owing to its significant presence in the erythropoietin (EPO) category with products such as Eprex and Neorecormon. Roche controls a substantial market share thanks to its extensively used Aranesp. Companies such as Novartis and Sanofi are aggressively investing in novel medicines for particular anemia subtypes, which might change the market landscape.
Market Dynamics
Market Growth Drivers
Demography and Disease Burden: Because of decreased nutritional absorption and an increasing frequency of chronic diseases, Brazil's rapidly aging population is particularly prone to anemia. This increasing category represents a significant market potential for anemia therapies. The increased prevalence of chronic illnesses such as renal failure, cancer, and autoimmune disorders frequently lead to secondary anemia, necessitating the development of specific therapy in addition to iron supplements.
Healthcare Transformation: Government investments in healthcare infrastructure, such as the Unified Health System (SUS), are increasing access to diagnosis and treatment, bringing more people into the system. Adoption of digital health technologies such as remote patient monitoring and telemedicine consultations can enhance anemia treatment and drug adherence, resulting in better clinical results.
Regulatory Policies: ANVISA's fast clearance procedure for novel anemia medications attracts multinational pharmaceutical manufacturers and promotes market competitiveness.
Market Restraints
Healthcare Infrastructure Limitations: Despite SUS advancements, healthcare infrastructure is still unevenly distributed, especially in rural and low-income regions. This restricts a large section of the population's access to prompt diagnosis, treatment, and vital anemia drugs. A shortage of specialist healthcare workers, particularly hematologists and oncologists, can result in delayed diagnoses, misdiagnoses, and inadequate treatment of severe anemia cases.
Affordability Issues and Cost Burdens: Certain novel anemia medicines, notably gene therapies and targeted pharmaceuticals, are expensive, making them difficult for both public healthcare systems and individual patients to afford, potentially restricting market adoption. Complexities and delays in insurance and government payment schemes can restrict patient access to vital pharmaceuticals, particularly for long-term therapies like erythropoietin-stimulating agents (ESAs). Lower-income people and families may struggle to buy even generic medications and supplements, resulting in an access gap and worsening existing healthcare inequities.
Market saturation: In the Brazil Anemia Therapeutics Market, dominant player influence is highly great. Established competitors with strong market positions, such as Cristalia Pharma and Roche, might make it difficult for emerging companies and novel cures to achieve momentum, thereby limiting market diversification.
Healthcare Policies and Regulatory Landscape
The Brazilian Health Regulatory Agency, often known as ANVISA, is in charge of regulating and overseeing a wide range of health goods and services, including medicines, medical devices, food, cosmetics, and tobacco. As the biggest country in South America and the second largest pharmaceutical market in the emerging world, Brazil's healthcare regulatory framework is critical to assuring the safety, efficacy, and quality of healthcare goods available to its nearly 200 million people. Anvisa's role is to promote public health by enforcing sanitary control over the manufacture, marketing, and use of health-regulated products and services, as well as associated settings, processes, substances, and technology. The agency is also involved in the approval, supervision, and inspection of drugs and clinical trials to be registered in Brazil, working in collaboration with other institutions such as CONEP (National Research Ethics Commission) and CEP (Research Ethics Committee) to ensure the ethical and regulatory compliance of clinical studies conducted in the country.
Competitive Landscape
Key Players:
- Amgen
- Roche
- Novartis
- Eli Lilly
- Sanofi
- Cristalia Pharma
- Ache Laboratories
- EMS Pharma
- Eurofarma
- BioLab Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Anemia Therapeutics Market Segmentation
By Type of Disease
- Iron Deficiency Anemia
- Megaloblastic Anemia
- Pernicious Anemia
- Hemorrhagic Anemia
- Hemolytic Anemia
- Sickle Cell Anemia
By Population
- Pediatrics
- Adults
- Geriatrics
By Therapy Type
- Oral Iron Therapy
- Parenteral Iron Therapy
- Red Blood Cell Transplantation
- Others
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Pharmacies
By End User
- In-Patient Centres
- Out-Patient Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.