Brazil Alzheimer’s Therapeutics Market Analysis
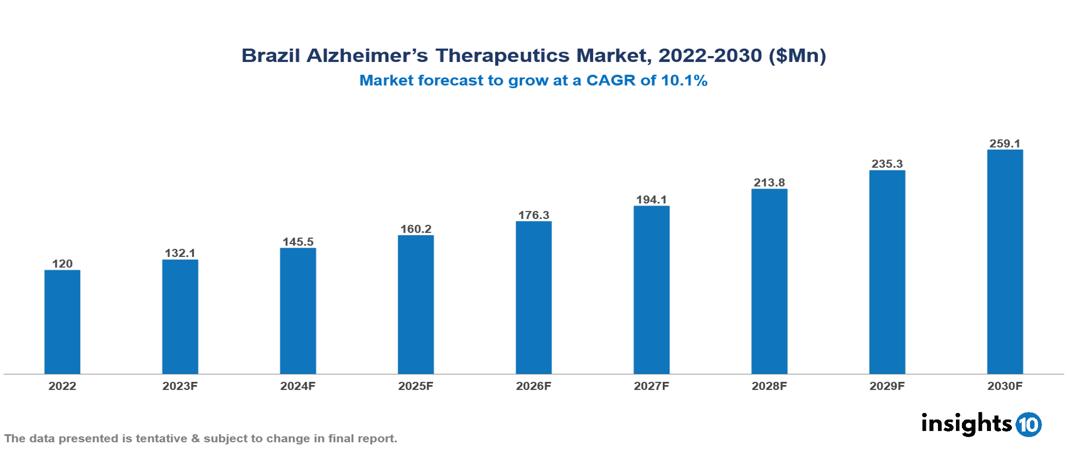
Brazil Alzheimer’s Therapeutics Market was valued at $120 Mn in 2022 and is estimated to reach $259 Mn in 2030, exhibiting a CAGR of 10.1% during the forecast period. One of the main factors propelling the market's growth is the rising demand for Alzheimer's treatment pharmaceuticals as a result of the increasing prevalence of Alzheimer's disease among the aging population. Leading pharmaceutical companies that are currently working in the market are Pfizer, Biogen, Janssen, Eisai, Novartis, Roche, Lundbeck, Otsuka Pharmaceutical, Teva Pharmaceutical and Sun Pharmaceutical.
Buy Now

Brazil Alzheimer’s Therapeutics Market Executive Summary
Brazil Alzheimer’s Therapeutics Market was valued at $120 Mn in 2022 and is estimated to reach $259 Mn in 2030, exhibiting a CAGR of 10.1% during the forecast period.
Alzheimer's is a neurological disorder that affects mental, behavioural, and memory abilities. Usually, it begins gradually, worsens over time, and makes it more difficult for the person to carry out everyday duties. In Alzheimer's disease, aberrant brain changes like plaque and tangle accumulation cause nerve cells to die. There is presently no treatment for Alzheimer's disease. Conversely, some medications can improve quality of life and help control symptoms. These drugs, which include memantine, rivastigmine, and donepezil, regulate certain neurotransmitters in the brain to treat memory and cognitive problems. In addition, there are non-pharmacological methods such as upholding a healthy lifestyle, participating in social and cognitive activities, and creating a supportive atmosphere that can enhance the overall management of sickness.
The incidence of dementia is 6.1% in Brazil, with significant age-group differences: 3.3% for people 60–64 years old and 43.0% for people 90 years of age and older. The prevalence of Alzheimer's disease is 313 cases per 100,000 people. While the prevalence rates show negative correlations with ultra-processed food consumption and physical activity levels, they also show favourable correlations with primary health care coverage-related characteristics. 98 cases of Alzheimer's disease occur for every 100,000 people. The death rate from the disease is positively correlated with the percentage of older people who are obese and who make up to half of the minimum wage, but it is inversely correlated with the percentage of older people who have diabetes-related factors.
Brazilian research facilities are actively involved in international clinical trials aimed at developing treatments for Alzheimer's disease. These studies involve the investigation of novel compounds that address different aspects of the disease, such as tau-targeting antibodies and BACE inhibitors.
Associação Brasileira de Alzheimer (ABRAZ) and Movimento Viva Bem com Alzheimer, are important Brazilian organizations that advocate for the early detection of Alzheimer's disease and raise awareness of the condition. The exchange of knowledge is facilitated by the joint efforts of pharmaceutical corporations, healthcare professionals, and patient advocacy groups, which eventually improves the quality of care rendered.
Market Dynamics
Market Growth Drivers
Increasing Aging Population: Brazil's population is aging faster than that of many other nations as a result of a demographic change. The demand for efficient treatment alternatives is expected to increase as the number of seniors increases and the prevalence of Alzheimer's disease rises.
Government Initiatives and Policies: Supportive policies and initiatives from the Brazilian government, like the National Alzheimer's Plan, are actively investing in research, diagnosis, and treatment of Alzheimer's disease. Government backing for research, healthcare infrastructure, and accessible treatment options can foster market growth.
Increased Awareness and Early Diagnosis: More people are seeking therapy for Alzheimer's because of increased public knowledge through proactive initiatives by organizations such as Associação Brasileira de Alzheimer (ABRAZ) and Movimento Viva Bem com Alzheimer of the illness and advancements in testing technology, which facilitate early diagnosis. The window of opportunity for potential improvement is extended when actions involving prospective disease-modifying medications are started early.
Market Restraints
Lack of Awareness and Stigma: Despite the growing number of awareness programs, many people are prevented from receiving a diagnosis and treatment for dementia and Alzheimer's disease due to the persistent societal stigma associated with these conditions. One significant barrier preventing the market from reaching a wider clientele is this stigma.
High Drug Costs and Affordability Concerns: The exorbitant expense of Alzheimer's therapy poses a significant obstacle to access for many sufferers. Given the current state of the insurance industry's coverage of certain pharmaceuticals, this issue is critical. Even with insurance, co-payments can put a heavy financial burden on patients and their families.
Limited Efficacy and Uncertain Long-Term Benefits: There are doubts about the long-term viability and efficacy of current medications, such as Leqembi and Aduhelm, in slowing down cognitive decline. Various factors contribute to hesitancy, such as the absence of a definitive treatment or conclusive evidence of better health.
Healthcare Policies and Regulatory Landscape
Agência Nacional de Vigilância Sanitária (ANVISA), the Brazilian Health Regulatory Agency, is in charge of overseeing treatment drug regulations and healthcare laws. Pharmaceutical companies need to submit extensive safety, effectiveness, and quality data for ANVISA approval in order to bring therapeutic medications, including Alzheimer's treatments, to market. ANVISA is in charge of managing the complete medication lifecycle, which includes pharmacovigilance efforts to guarantee continued safety, post-marketing surveillance, and analysis of clinical trial outcomes. Sistema Único de Saúde (SUS), the public healthcare system run by the Brazilian government, sets reimbursement guidelines that affect the availability and cost of treatment medications. The rights of pharmaceutical businesses are also protected by intellectual property regulations.
Competitive Landscape
Key Players
- Pfizer
- Biogen
- Janssen
- Eisai
- Novartis
- Roche
- Lundbeck
- Otsuka Pharmaceutical
- Teva Pharmaceutical
- Sun Pharmaceutical
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Alzheimer’s Therapeutics Market Segmentation
By Type
- Early-Onset Alzheimer's
- Late-Onset Alzheimer's
- Familial Alzheimer's disease
By Drug Name
- Donepezil
- Rivastigmine
- Memantine
- Galantamine
- Manufactured a combination of memantine and donepezil
By Drug Class
- Cholinesterase Inhibitors
- NMDA Receptor Antagonists
- Manufactured Combination
By End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
By Distribution Channel
- Hospital pharmacies
- Drug stores
- Retail pharmacies
- Online pharmacies
- Other distribution channel
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.