Brazil Age-Related Macular Degeneration Therapeutics Market Analysis
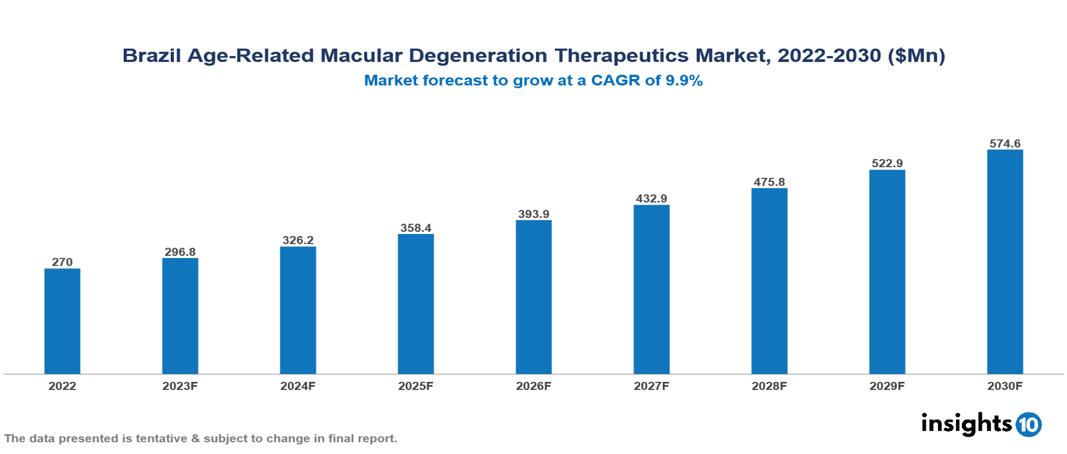
The Brazil Age-Related Macular Degeneration (AMD) Therapeutics Market was valued at US $270 Mn in 2022 and is predicted to grow at a CAGR of 9.9% from 2023 to 2030, to US $575 Mn by 2030. The key drivers of this industry include the upward trend in the prevalence of age-related macular degeneration, the evolving healthcare landscape, and other factors. The industry is primarily dominated by players such as Regeneron Pharmaceuticals, Novartis, Bayer, Roche, and Genetech, among others
Buy Now

Brazil Age-Related Macular Degeneration Therapeutics Market Analysis: Executive Summary
The Brazil Age-Related Macular Degeneration (AMD) Therapeutics Market is at around US $270 Mn in 2022 and is projected to reach US $575 Mn in 2030, exhibiting a CAGR of 9.9 % during the forecast period.
Age-Related Macular Degeneration (AMD) is a gradual eye condition primarily affecting older individuals, potentially resulting in significant vision impairment. It impacts the macula, responsible for central vision, making tasks like reading challenging. AMD manifests in two main types, which are dry AMD and wet AMD. Symptoms include distorted vision, difficulty seeing clearly, and a gradual decline in eyesight. Dry AMD is more common, but it progresses more slowly. Conversely, wet AMD, though less prevalent, is more severe and is characterized by abnormal blood vessel growth beneath the macula. Treatment options encompass anti-VEGF therapy, including medications like Lucentis, Vabysmo, Avastin (manufactured by Genentech), and Eylea (by Regeneron Pharmaceuticals), along with laser therapy and nutritional supplements like zinc and copper, which are more preventive in nature.
It is estimated that the prevalence of AMD is around 11.5% among individuals aged 50 and older, approximately 15% in those aged 60, and about 31% in the demographic aged 80 and above in Brazil. Thus, the prevalence is directly proportional to age. The market is therefore driven by major factors like the increase in the aging population and subsequent increase in prevalence, technological advancements in developing new therapies, and the evolving healthcare landscape of the country propelling the therapeutics industry. However, disparities in the healthcare system and the high expense of therapies like gene therapy and laser surgery, which are adopted in response to demand, limit the market's potential.
The market leader in Brazil is Roche, with Lucentis earning a market share of around 40%, closely followed by Novartis for both Lucentis and a low-cost alternative, Avastin, with a share of about 25% in the market.
Market Dynamics
Market Growth Drivers
Rising prevalence of AMD: Brazil possesses a substantial population aged 65 and above, the primary demographic vulnerable to AMD, and projections indicate continuous growth in the years ahead. This increasing elderly population results in a larger potential patient pool, driving demand in the market. The age-standardized prevalence of AMD is estimated to be around 11.5% in people greater than 50 years, around 15% in people aged 60 years, and approximately 31% in the age group above 80 years.
Evolving healthcare landscape: Initiatives by the government, such as the National Eye Health Program and the broadening of health insurance coverage for specific AMD treatments, play a role in enhancing the accessibility and affordability of therapies, and fostering growth in the market. The growing collaborations between pharmaceutical firms, research institutions, and healthcare providers through increased public-private partnerships provide a collaborative approach to advancing AMD care and expanding the market's reach.
Technological advancements: Innovation in the field of AMD therapies is on the rise, with notable advancements in stem cell and gene therapy as well as the emergence of anti-VEGF medications offering promising new avenues for treatment. Additionally, investment in clinical trials and local research and development fosters the market for growth.
Market Restraints
Health system disparities: There exist inequities in the level and type of care available between different regions of Brazil. Major cities are focal points for specialized ophthalmologists and treatment facilities, creating a disparity in healthcare services with rural areas being underserved. This geographical divide results in delayed diagnosis, restricted treatment choices, and poorer outcomes for patients in rural areas.
High cost of treatments: The conventional treatment for wet AMD, which involves anti-VEGF injections, is expensive. The expense for a vial of Lucentis solution is approximately $1200 for patients who pay in cash. This imposes a considerable financial strain on both the healthcare system and individual patients. Additionally, SUS insurance does not cover these costs, establishing a financial and accessibility obstacle for patients.
Healthcare Policies and Regulatory Landscape
Brazil's healthcare regulatory framework is overseen by various entities. The National Supplementary Health Agency (ANS) regulates the private healthcare system, establishing primary rules for healthcare plan operations and monitoring their functions. The Ministry of Health regulates the public healthcare system, playing a significant role in overseeing and promoting the expansion of healthcare services at the municipal level. Additionally, the medical device market in Brazil is governed by the Agência Nacional de Vigilância Sanitária (ANVISA), which has made significant strides in aligning medical device registration requirements with international standards.
To obtain healthcare licensure in Brazil, navigating the regulations set by ANVISA for medical devices and the ANS for healthcare plans is essential. For medical devices, companies must comply with requirements outlined in relevant resolutions. Regarding healthcare plans, entities must adhere to rules established by the ANS for the ongoing provision of healthcare services. Additionally, foreign companies need to have a local importer or distributor and ensure that their products meet the quality and regulatory standards in Brazil.
Competitive Landscape
Key Players
- F. Hoffman-La-Roche Ltd
- Regeneron Pharmaceuticals
- Novartis AG
- Apellis Pharmaceuticals
- Neurotech Pharmaceuticals
- Bayer
- Pfizer Inc
- Genetech
- Kanghong Pharma
- Bausch Health Companies, Inc
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Brazil Age-Related Macular Degeneration Therapeutics Market Segmentation
By Disease Type
- Dry AMD
- Wet AMD
By Drug
- Lucentis
- Eylea
- Beovu
By Age Group
- Less than 60
- Between 60-80
- More than 80
By Stage
- Early AMD
- Intermediate AMD
- Advanced AMD
- No AMD
By Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.