Australia Cancer Immunotherapy Market Analysis
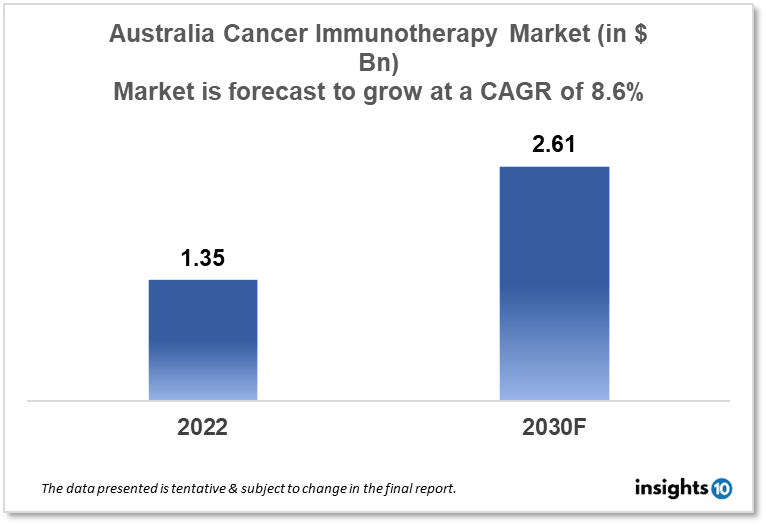
Australia's cancer immunotherapy market is expected to grow from $1.35 Bn in 2022 to $2.61 Bn in 2030 with a CAGR of 8.6% for the forecasted year 2022-30. The rising incidence of cancer cases and supportive government programs to promote cancer immunotherapy in Australia are responsible for the growth of the market. The Australian cancer immunotherapy market is segmented by type, application, and end user. Bioarc, Pharmaxis, and Bristol-Myers Squibb are the major players in the Australian cancer immunotherapy market.
Buy Now

Australia Cancer Immunotherapy Market Executive Analysis
Australia's cancer immunotherapy market is expected to grow from $1.35 Bn in 2022 to $2.61 Bn in 2030 with a CAGR of 8.6% for the forecasted year 2022-30. To support the proposals of the Strengthening Medicare Taskforce, the government has set up 750 Mn dollars for the Strengthening Medicare Fund. We are happy to see that the government intends to proceed swiftly with the distribution of $229.7 Mn in General Practitioner (GP) infrastructure grants of up to $50,000 apiece, which will aid general practices in improving their digital capacity, making investments in infection control, and meeting accreditation standards. The government's $143.3 Mn pledge to promote healthcare access in rural and regional areas is also welcomed by the American Medical Association (AMA). Any additional federal funding will simply be devoured by rising healthcare expenses before it even reaches a single patient, leaving no funding to provide additional services to address community health needs. Inflation is predicted to reach 7.75 % by the December quarter of this year.
In Australia, there were 48,266 cancer-related fatalities in 2020 (27,167 men and 21,099 women). A projected 49,996 people will pass away in 2022 (28,022 men and 21,974 women). A person's risk of dying from cancer by the age of 85 is predicted to be 1 in 7 (or 15%) in 2022 (1 in 6 or 17% for men and 1 in 8 or 13% for women).
There are various forms of immunotherapy, and each one functions differently. Immunotherapy can improve the immune system's ability to fight cancer or lower obstacles in the way of the immune system's immunological onslaught. The immune system can be prevented from attacking cancer cells by 'checkpoints', proteins that are found on the surface of T-cells. Drugs called checkpoint inhibitors are made to stop these proteins so that the T-cells can recognize and eliminate cancer cells. The most popular type of immunotherapy now uses these medications. The Pharmaceutical Benefits Scheme (PBS) offers subsidies for some. Some immunotherapy regimens are intended to reawaken and combat cancer cells by stimulating the immune system. The use of CAR (chimeric antigen receptor) T-cell therapy increases T-cells' capacity to combat malignancy. Before being reintroduced into the circulation via an intravenous drip, these cells are taken from the blood and modified to better recognize cancer cells. Clinical trials are examining whether CAR T-cell therapy is effective for treating different cancer types in addition to leukaemia and lymphoma that it is used to treat in some cases. Utilizing viruses that infect cancer cells and kill them while activating the immune system to fight the disease, this treatment is known as oncolytic virus therapy. Oncolytic virus therapies are being tested in clinical trials for brain cancer and a few other tumours, and this therapy is occasionally used to treat melanoma. The research is still in its early stages.
Market Dynamics
Market Growth Drivers
With more than 150,000 new cases of cancer identified each year, Australia is a country with a significant cancer epidemic. The need for potent therapies like immunotherapy is anticipated to increase as cancer incidence rates keep rising. The use of immunotherapy is expanding quickly in Australia as a viable treatment option for a variety of cancers. The demand for these treatments is anticipated to increase as more medical professionals and patients become aware of the advantages of immunotherapy. The Australian government has put in place a number of programs to aid in the creation and marketing of cancer immunotherapies. These include of providing money for research and development, streamlining the regulatory system, and paying for approved medical procedures. The Australian government and a number of private groups have made significant investments in cancer research, including studies into immunotherapy. This has sparked the creation of novel medicines and the improvement of current ones, both of which are anticipated to spur the Australian cancer immunotherapy market expansion.
Market Restraints
Immunotherapy treatments are only partially covered by the Australian government, which may restrict patient's access to these medicines. Immunotherapy treatments have the potential to be very beneficial, but they also carry a risk of serious adverse effects, some of which might be life-altering for certain patients. The number of patients who are able or willing to receive these treatments may be constrained as a result. Chemotherapy and radiation therapy are two more cancer treatments that may compete with immunotherapy treatments for market share which can limit the growth of Australia's cancer immunotherapy market.
Competitive Landscape
Key Players
- Luina Bio (AUS)
- Westlab (AUS)
- Telix Pharmaceuticals (AUS)
- Bioarc (AUS)
- Pharmaxis (AUS)
- Bristol-Myers Squibb
- Eli Lily
- F. Hoffmann-La Roche
- Pfizer
- Johnson & Johnson
- Merck
- Novartis
Healthcare Policies and Regulatory Landscape
The government-funded health insurance program Medicare, which offers access to a variety of medical services, including cancer treatments like chemotherapy, radiation therapy, and surgery, covers a major portion of the cost of cancer treatment in Australia. There are private health insurance choices available in addition to Medicare, which can offer extra coverage for cancer treatments, such as access to private hospital rooms or more specialized care. Private health insurance, however, can be pricey and not available to all individuals. Drugs must fulfil specific requirements, such as being accepted by the Therapeutic Goods Administration (TGA), the Australian government body in charge of overseeing medications and medical devices, in order to qualify for PBS subsidies. The Medical Benefits Schedule (MBS), a different program run by the Australian government, offers compensation for medical operations and services, including cancer treatments like radiation therapy and surgery. Patients can be required to pay out of pocket for some cancer treatments because not all cancer therapies are covered by the PBS or MBS. Furthermore, some treatments might only be accessible through clinical trials or as a part of programs for compassionate use, which might not be reimbursed by insurance or supported by the government.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Cancer Immunotherapy Segmentation
By Type (Revenue, USD Billion):
- Monoclonal Antibodies
- Cancer Vaccines
- Checkpoint Inhibitors
- Immunomodulators
- PD-1/PD-L1
- CTLA-4
By Application (Revenue, USD Billion):
- Lung Cancer
- Breast Cancer
- Head and Neck Cancer
- Prostate Cancer
- Colorectal Cancer
- Melanoma
- Others
By End User (Revenue, USD Billion):
- Hospitals
- Clinics
- Ambulatory Surgical Centers (ASCs)
- Cancer Research Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
Bioarc, Pharmaxis, and Bristol-Myers Squibb are the major players in the Australia cancer immunotherapy market.
The Australia cancer immunotherapy market is expected to grow from $1.35 Bn in 2022 to $2.61 Bn in 2030 with a CAGR of 8.6% for the forecasted year 2022-2030.
The Australia cancer immunotherapy market is segmented by type, application, and end user.









































