Algeria Neurology Devices Market Analysis
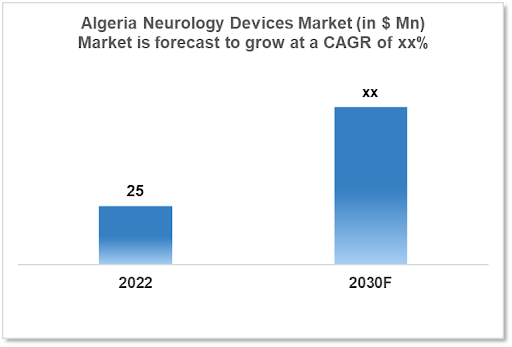
The Algeria Neurology Device Market is expected to witness growth from $25 Mn in 2022 to $xx Mn in 2030 with a CAGR of xx% for the forecasted year 2022-2030. The Prevalence of Neurological illnesses has increased in Algeria, which is increasing the demand for neurology devices. The population is ageing, habits are changing, and there are environmental issues to blame for this. The market is segmented by product type and by the end user. Some key players in this market include: Techno Brain Group, Biodeal Laboratories, Johnson and Johnson, GE Healthcare, Stryker, Medtronic and Abbott laboratories
Buy Now

Algeria Neurology Devices Market Executive Analysis
The Algeria Neurology Devices Market size is at around $25 Mn in 2022 and is projected to reach $xx Mn in 2030, exhibiting a CAGR of xx% during the forecast period. Algeria spent about 6.6% of its GDP on healthcare in 2021. In Algeria, the cost of healthcare is $260 per person, while public health spending is $176 per person. In Algeria, there were roughly 1.63 strokes per 1000 people per year. In Algeria, the prevalence of active epilepsy was reported to be 5.2 per 1000 people. Epilepsy is also a common neurological disorder in Algeria. The prevalence of multiple sclerosis (MS), another widespread neurological disorder in Algeria, was 4.4 per 100,000 people in 2020. The prevalence of Parkinson's disease is 89 per 100,000 people in Algeria, making it a common neurological disease.
Parkinson's disease, epilepsy, traumatic brain injury, Alzheimer's disease, major depressive disorder, and spinal cord injury are all treated with neurology devices. Neurology devices can be used to restore hearing and vision, as well as to improve functioning for amputees or those with congenital limb differences. Sacral nerve stimulation devices are frequently used by patients who don't respond well to pharmaceutical therapy or other types of medication. The sacral nerves in the lower back that are being treated are stimulated with electrical impulses using a modest instrument. Patients with severe brain injuries or other disorders requiring ongoing vital sign monitoring are monitored with neurology devices including intracranial pressure monitors and electrocardiograms. These devices make it easier for healthcare providers to spot and respond to changes in a patient's condition promptly.
Market Dynamics
Market Growth Drivers
The Prevalence of Neurological illnesses has increased in Algeria, which is increasing the demand for neurology devices. The population is ageing, habits are changing, and there are environmental issues to blame for this. The creation of more sophisticated and advanced neurology devices, including implanted devices, neurostimulation devices, and improved imaging systems, is a result of technological advancements. Due to their improved diagnostic and therapeutic capabilities, these devices are in more demand on the market.
Market Restraints
Algeria's market for neurology devices is getting more and more cutthroat as local and foreign firms compete for market dominance. Because of this, it could be challenging for manufacturers to stand out and take control of the Algeria neurology devices market. Manufacturers of neurology devices may find it challenging to get their devices certified for usage on the market in Algeria due to the country's complicated regulatory environment. Delays in product introductions and increased expenses for manufacturers may result from this.
Competitive Landscape
Key Players
- Mediline (DZ)
- SODIMED (DZ)
- Medtronic
- Abbott Laboratories
- Johnson & Johnson
- Boston Scientific
- GE Healthcare
Healthcare Policies and Regulatory Landscape
The Ministry of Health, Population, and Hospital Reform (MSPRH) oversees Algeria's healthcare regulations and market for neurology devices. The MSPRH is in charge of creating and enforcing the laws and regulations that control the importation, distribution, and sale of neurology devices in Algeria. Neurology device is categorised in Algeria according to their intended application, degree of danger, and legal restrictions. The International Medical Device Regulators Forum (IMDRF) framework, which offers a unified, global approach to neurology device regulation, serves as the foundation for classifying neurology devices. Manufacturers of neurological devices in Algeria are required to follow Good Manufacturing Practice (GMP) regulations in addition to registration. The GMP specifications are designed to guarantee that neurological equipment is produced in secure, efficient, and of the highest calibre ways.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Neurology Device Market Segmentation
The Neurology Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Neurostimulation
- ?Spinal Cord Stimulation Devices
- Deep Brain Stimulation Devices
- Sacral Nerve Stimulation
- Vagus Nerve Stimulation
- Gastric Electric Stimulation
- Interventional Neurology
- Aneurysm Coiling & Embolization
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Cerebral Balloon Angioplasty & Stenting
- Carotid Artery Stents
- Filter Devices
- Balloon Occlusion Devices
- Neurothrombectomy
- Clot Retriever
- Suction Aspiration Devices
- Snares
- CSF Management
- CSF Shunts
- CSF Drainage
- Neurosurgery Devices
- Ultrasonic Aspirators
- Stereotactic Systems
- Neuroendoscopes
- Aneurysm Clips
By End User (Revenue, USD Billion):
- ??Hospitals and Clinics
- Specialty Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































